
U.S. Department of Health and Human Services
Assistant Secretary for Planning and Evaluation
Office of Health Policy
Data Sources and Data-Linking
Strategies to Support Research to
Address the Opioid Crisis
FINAL REPORT
September 2018

The Office of the Assistant Secretary for Planning and Evaluation (ASPE) is the
principal advisor to the Secretary of the Department of Health and Human Services
(HHS) on policy development issues, and is responsible for major activities in the areas
of legislative and budget development, strategic planning, policy research and
evaluation, and economic analysis.
The Office of Health Policy (HP), within ASPE, provides a cross-cutting policy perspective
that bridges Departmental programs, public and private sector activities, and the research
community, in order to develop, analyze, coordinate and provide leadership on health
policy issues for the Secretary.
This report was prepared under contract # HHSP23320095649WC. The task order number
for the current Time & Materials umbrella contract is: HHSP23337038T between HHS’s
ASPE/HP and the RAND Corporation.
The opinions and views expressed in this report are those of the authors. They do not necessarily
reflect the views of the Department of Health and Human Services, the contractor or any other
funding organization.

September 2018
Data Sources and Data-Linking Strategies to
Support Research to Address the Opioid Crisis
Final Report
Submitted to
Office of Health Policy
Assistant Secretary for Planning and Evaluation
U.S. Department of Health and Human Services
Hubert H. Humphrey Building
200 Independence Avenue SW
Washington, DC 20201
Submitted by
Rosanna Smart, Courtney Ann Kase, Amanda Meyer, and Bradley D. Stein
RAND Corporation
1776 Main Street
P.O. Box 2138
Santa Monica, CA 90407-2138

About This Report
This report presents findings from a scoping study to assess the types of data sources and
data-linkage efforts that are currently being used or could potentially be leveraged to support
research and evaluations relevant to the U.S. Department of Health and Human Services
Strategic Priorities to combat the opioids crisis. Based on an environmental scan of the literature
and interviews with opioid policy and research efforts, the purpose of the project is to provide an
overview of the types of secondary data sources and data linkages commonly used in opioid-
related research to highlight some of the key gaps or challenges for existing data-collection and
analysis efforts and to outline potential steps that could be taken to overcome these challenges.
The initial scoping study was conducted in summer 2017, with an update to the scan of the
literature conducted in February 2018.
We would like to acknowledge the participation and assistance of all researchers and federal
program officials who participated in the stakeholder interviews. This effort would not have been
possible without their generosity in providing their time and expertise on challenges and
opportunities for the use of secondary data in research relevant to the opioids crisis. We also
thank Hilary Peterson and Mary Vaiana for their keen attention to detail and for providing
excellent assistance in the creation of this report. Finally, we would like to acknowledge the
contributions of Susan Lumsden and Scott R. Smith from the Office of the Assistant Secretary
for Planning and Evaluation, as well as the valuable insights we received from the peer reviewers
of the report, Erin Taylor of RAND and Brendan Saloner of Johns Hopkins University.
The research reported here was undertaken within RAND Health, a division of the RAND
Corporation, and funded by the Office of the Assistant Secretary for Planning and Evaluation,
Department of Health and Human Services. A profile of RAND Health, abstracts of its
publications, and ordering information can be found at www.rand.org/health. Questions and
comments about this report should be sent to the project leader, Bradley Stein ([email protected]).
About the Authors
Bradley Stein is a senior physician policy researcher at the RAND Corporation and an adjunct
associate professor of psychiatry at the University of Pittsburgh School of Medicine. A
practicing psychiatrist and health services and policy researcher, his research is focused on better
understanding and improving care for individuals with mental health and substance use disorders
in community settings.
ii
Rosanna Smart is an associate economist at the RAND Corporation whose research centers on
studying the public health and policy implications of licit and illicit substance use, drug markets
and drug policy, and issues related to the criminal justice system.
Courtney A. Kase is a policy analyst at the RAND Corporation whose prior research includes
evaluations of service integration within community-based behavioral health centers, approaches
to reducing health disparities, and approaches for technology use and collaboration in rural
educational settings.
Amanda Meyer is a research assistant at the RAND Corporation with research interests in
tobacco control and regulation, mental health policy and interventions, trauma, and school
health.
iii

Contents
About This Report ......................................................................................................................ii
Tables......................................................................................................................................... v
Abbreviations ............................................................................................................................ vi
1. Introduction ............................................................................................................................ 1
2. Background on the U.S. Department of Health and Human Services’ Strategic Priorities........ 3
Better Practices for Pain Management............................................................................................. 3
Better Addiction Prevention, Treatment, and Recovery Services...................................................... 4
Better Targeting of Overdose-Reversing Drugs ............................................................................... 4
Better Data...................................................................................................................................... 5
Better Research............................................................................................................................... 5
3. Current State of the Evidence: Findings from the Environmental Scan.................................... 8
Better Practices for Pain Management............................................................................................. 8
Better Addiction Prevention, Treatment, and Recovery Services.................................................... 11
Better Targeting of Overdose-Reversing Drugs ............................................................................. 13
Better Data.................................................................................................................................... 15
4. Sources of Secondary Data: Data Inventory Findings............................................................ 17
National Surveys........................................................................................................................... 19
Electronic Health Records and Claims Data .................................................................................. 20
Mortality Records ......................................................................................................................... 22
Prescription Drug–Monitoring Data .............................................................................................. 22
Contextual and Policy Data........................................................................................................... 23
Other National, State, and Local Sources....................................................................................... 24
5. High-Priority Research Needs and Data Efforts: Findings from the
Stakeholder Discussions ..................................................................................................... 26
Better Practices for Pain Management........................................................................................... 26
Better Addiction Prevention, Treatment, and Recovery Services.................................................... 30
Better Targeting of Overdose-Reversing Drugs ............................................................................. 34
Better Data.................................................................................................................................... 36
6. Challenges and Opportunities for Implementing Successful Data-Linking Strategies ............ 40
Summary ...................................................................................................................................... 49
References................................................................................................................................ 52
iv
Appendix Overview of Types of Secondary Data Sources and Data Inventory Content 72— ..........

Tables
v
Table 3.1. Commonly Used Data Sources and Measures in Research to Advance Better Pain
Management Practices ......................................................................................................... 9
Table 3.2. Contextual Data Sources and Measures Commonly Linked to Opioid Outcome Data in
Research Related to the Five-Point HHS Strategy.............................................................. 10
Table 3.3. Commonly Used Data Sources and Measures in Research to Improve Addiction
Prevention, Treatment, and Recovery Services .................................................................. 12
Table 4.1. Data Source Categories Identified ............................................................................. 18
Table 4.2. Comparison of Electronic Health Record and Administrative Claims Data ............... 21
Table 5.1. Commonly Referenced Data Sources for Understanding Better Practices for Pain
Management...................................................................................................................... 28
Table 5.2. Commonly Referenced Data Sources for Understanding Treatment Need and Access
.......................................................................................................................................... 32
Table 5.3. Commonly Referenced Data Sources for Understanding Naloxone Access ............... 35
Table 5.4. Commonly Referenced Data Sources for Understanding the Epidemic Through Better
Public Health Surveillance ................................................................................................ 38
Table 6.1. Time Frame for Potential Approaches to Implementing Successful Data-Linking
Strategies .......................................................................................................................... 50
Table A.1. National Survey Data ............................................................................................... 75
Table A.2. Claims and Electronic Health Records Secondary Data Sources .............................. 78
Table A.3. Mortality Records .................................................................................................... 84
Table A.4. Prescription Monitoring Secondary Data Sources .................................................... 86
Table A.5. Contextual and Policy Data Sources ........................................................................ 89
Table A.6. Other National, State, and Local Secondary Data Sources ........................................ 91

Abbreviations
ADAM Arrestee Drug Abuse Monitoring
AHRQ Agency for Healthcare Research and Quality
ARCOS Automation of Reports and Consolidated Orders System
CDC Centers for Disease Control and Prevention
CMS Centers for Medicare and Medicaid Services
DAWN Drug Abuse Warning Network
DEA Drug Enforcement Agency
DEA ACSA Drug Enforcement Agency Active Controlled Substances Act Registrants
Database
EHR electronic health record
EMS Emergency medical services
HHS Department of Health and Human Services
MEPS Medical Expenditure Panel Survey
NAMSDL National Alliance for Model State Drug Laws
NAVIPPRO National Addictions Vigilance Intervention and Prevention Program
NDI National Death Index
NEMSIS National Emergency Medical Services Information System
NESARC National Epidemiologic Survey on Alcohol and Related Conditions
NPDS National Poison Data System
NSDUH National Survey on Drug Use and Health
N-SSATS National Survey of Substance Abuse Treatment Services
NVSS MCOD National Vital Statistics System Multiple Cause of Death
OEND overdose education and naloxone distribution
PBSS Prescription Behavior Surveillance System
PDAPS Prescription Drug Abuse Policy System
PDMP prescription drug monitoring program
RADARS Researched Abuse, Diversion and Addiction-Related Surveillance System
SAMHSA Substance Abuse and Mental Health Administration
STRIDE System to Retrieve Information from Drug Evidence
TEDS Treatment Episodes Data Set
vi
1. Introduction
The Department of Health and Human Services
(HHS) has a five-point strategy for addressing the
significant social and public costs associated with
the opioid crisis (see Box 1) (HHS, undated).
Numerous efforts are underway to implement these
strategies, which are intended to address key
contributors and harms related to the opioid crisis,
enhance the ability of public health officials and
policymakers to monitor the crisis as it evolves,
and facilitate more-informed policymaking.
However, progress will also be made by identifying
which research questions to prioritize, data sources
to support such research, and approaches that can be used to leverage or link multiple
complementary data sources. Much of the research on the opioid crisis relies on information
drawn from sources outside of clinical research settings. Researchers can leverage “real-world
evidence” to enhance the field’s ability to address the crisis and generate new evidence to inform
decisions.
Box 1. HHS Strategic Priorities
! Better practices for pain
management
! Better addiction prevention,
treatment, and recovery services
! Better targeting of overdose-
reversing drugs
! Better data
! Better research.
The ability to link data—combining data from two or more sources to study the same
individual, facility, organization, e vent, or geographic area—often makes it possible to enhance
the value of the information obtained beyond what is available from any single source. Data sets
that contain unique individual identifiers make it possible to link information from different
sources at the individual level. Linkages at a more-aggregate level include analyses that merge
two or more data sources at the state or county level or at a finer geographic level. Finally, while
they do not directly “link” data sources, many studies analyze multiple complementary data
sources (e.g., geographic spatial analyses of heroin-related emergency department visits and
heroin-related deaths) to provide more-robust or comprehensive evidence of policy or program
impact (Hudson, Klekamp, and Matthews, 2017). Each method has strengths and limitations, but
all can contribute toward informing evidence-based policymaking (Commission on Evidence-
Based Policymaking, 2017).
This report provides an overview of the types of secondary data sources currently being used
or that could potentially be used to evaluate interventions or conduct other analyses that address
the five-part HHS strategy. The report highlights key research questions in each area and
identifies opportunities to use existing data sources and implement data-linking strategies that
can support assessments of the HHS strategy. Findings are based on interviews with 16
experts—academic researchers, federal researchers, and federal program officials—
1
complemented by an environmental scan of the literature. This report does not address all the
strengths and limitations of these data sources; rather, it is intended to provide sufficient
information to serve as a resource to researchers in the field of opioids and opioid use disorder.
This report is organized as follows:
• Chapter 2 provides background information on each of the HHS Strategic Priorities.
• Chapter 3 informs the Strategic Priority of better research by presenting an overview of
existing research related to the first four HHS Strategic Priorities as identified through an
environmental scan, including commonly used data sources and common approaches to
linking or merging data sources.
• Chapter 4 broadly categorizes the types of secondary data sources used in research
related to the Strategic Priorities and provides examples of specific data sources and data
elements.
• Chapter 5 describes findings identified through stakeholder discussions on key research
needs and the opportunities and challenges for using secondary data sources to address
those needs.
• Chapter 6 summarizes key challenges facing researchers and policymakers in studying
and responding to the opioid crisis and suggests potential solutions.
2

2. Background on the U.S. Department of Health and Human
Services’ Strategic Priorities
Addressing the opioid crisis is one of HHS’s top priorities. Therefore, HHS has developed a
comprehensive strategy to empower local communities on the frontlines. In 2017, HHS unveiled
a five-point strategy, encompassing (1) better pain management; (2) better treatment, prevention,
and recovery services; (3) better targeting of overdose-reversing drugs; (4) better data on the
crisis; and (5) better research to inform strategies to combat the crisis. In this chapter, we provide
an overview of information needs and research considerations underlying each component of the
strategy.
Better Practices for Pain Management
An estimated 20 percent of noncancer outpatients with pain receive opioid analgesics
(Daubresse et al., 2013); those who receive such medications chronically are at significant risk of
developing an opioid use disorder (Boscarino et al., 2010), characterized by persistent use that is
functionally impairing (American Psychiatric Association, 2013). Growth in opioid analgesic
prescribing has occurred alongside increasing rates of opioid-related misuse, emergency
department visits, and deaths (HHS, 2013; Rudd et al., 2016). Efforts to minimize opioid-
prescribing practices that likely lead to misuse or opioid-related harms must be balanced with
maintaining appropriate, high-quality pain management for patients (Interagency Pain Research
Coordinating Committee, 2015).
In recent years, federal agencies such as the Centers for Disease Control and Prevention
(CDC) and Centers for Medicare and Medicaid Services (CMS) have worked with private
insurers, medical educators, and other stakeholders to promote safe opioid use while limiting
addiction risk (Price, 2017). National medical organizations, states, and large health systems
have published clinical practice guidelines for prescribing opioids for chronic pain (Nuckols et
al., 2014; Haegerich et al., 2014; Mai et al., 2015). Likewise, efforts by the Interagency Pain
Research Coordinating Committee (created by HHS) and CDC have worked toward providing
clinicians, researchers, and the public with recommendations concerning the prescribing and use
of opioids for pain management (Interagency Pain Research Coordinating Committee, 2015;
Dowell, Haegerich, and Chou, 2016). Federal agencies have also called for research and science
to improve the effectiveness of existing alternative pain treatments, including nonpharmacologic
options (e.g., physical or behavioral therapy) and nonopioid pharmacotherapies, and to develop
treatments for pain that are safer and more effective than opioid analgesics (Volkow and Collins,
2017). While research in this area continues to develop, important questions remain about how
3
pain can be treated more effectively while minimizing potential unintended consequences such
as dependence and overdose.
Better Addiction Prevention, Treatment, and Recovery Services
Opioid use disorders, which, in 2016, affected over 2.1 million people in the United States
(Amhsbrak et al., 2017), contribute to medical morbidity, can promote risky behaviors, and often
complicate treatment for human immunodeficiency virus (HIV) and other comorbid conditions
(Becker et al., 2007; Becker et al., 2008; Johnson et al., 2013; Broz and Ouellet, 2008; CDC,
2012; Hall et al., 2008; Estrada, 2005). The availability of medication-assisted therapies has been
substantially improved in part because of collaborations between HHS agencies and public and
private stakeholders (Volkow et al., 2014), however, substantial gaps persist between the need
for treatment and the capacity to provide it (Saloner and Karthikeyan, 2015; Jones et al., 2015;
Feder, Krawczyk, and Saloner, 2017; Morgan et al., 2018; Hadland, Wharam, and Schuster,
2017). Thus, there is a critical need to better understand and address existing provider, patient,
and systemic barriers to treatment (Chou, Korthuis, and Weimer, 2016; Rinaldo and Rinaldo,
2013; Shen and Zuckerman, 2005; Cunningham and Nichols, 2005; Bradley, Dahman, and
Given, 2009; Schuur et al., 2009; Yoo et al., 2010; Kwiatkowski et al., 2000; Maddux and
Desmond, 1997; Clark et al., 2011; Burns et al., 2016) to improve access to treatment (Watkins
et al., 2017) and recovery services, and to ensure high-quality care (Chou, Korthuis, and Weimer,
2016; Gordon et al., 2016). To promote evidence-based prevention and treatment activities, $485
million in grants were distributed in 2017 to states through the 21st Century Cures Act, with
additional grants forthcoming based on further assessment of effective strategies and community
needs (Price, 2017).
Better Targeting of Overdose-Reversing Drugs
In 2016, more than 42,000 overdose deaths involved opioids; nearly 40 percent involved
heroin (Rudd et al., 2016; National Institute on Drug Abuse, 2017; CDC, 2017) and almost 45
percent involved synthetic opioids (e.g., fentanyl) (CDC, 2017). Overdose deaths often involved
multiple opioids or other medications such as benzodiazepines. Overdose-reversing drugs, such
as naloxone, play a critical role in preventing opioid overdose death. With the emergence of new
formulations of naloxone that can more easily be administered by individuals without medical
training (Merlin et al., 2015; Gupta, Shah, and Ross, 2016), efforts to encourage naloxone access
and use have grown rapidly, generally through three broad mechanisms: (1) community-based
distribution programs to expand community access to naloxone (Wheeler et al., 2015; Fairbairn,
Coffin, and Walley, 2017), (2) state laws and protocols encouraging bystanders to summon first
responders in the event of an overdose (Davis and Carr, 2015) and broadening the authority of
emergency services personnel and other first responders (e.g., law enforcement) to administer
naloxone (Davis, Southwell et al., 2014; Davis, Ruiz et al., 2014), and (3) policies to encourage
4
retail pharmacy dispensing of naloxone (Davis and Carr, 2017). Given the continued growth in
opioid-overdose mortality and influx of lethal synthetic opioids, promoting access to and use of
overdose-reversing drugs is essential to combat this public health crisis (Price, 2017).
Better Data
To understand effective strategies to reduce opioid misuse and associated harms and monitor
the evolving crisis, data are needed that can capture trends in opioid use, risk or protective
factors that influence the transition to risky use or opioid use disorder, and the risk among opioid
users of experiencing mortality or other harms. Given the rapidity with which opioid use and
markets have evolved over the past decade, developing and using public health surveillance
systems that offer near-real-time information have become essential. Historically, death
certificate and hospitalization data have been used to monitor drug use trends, but these sources
often suffer from data availability lags of one or two years. Variation in medical examiner and
coroner procedures in determining manner of death and the specific drugs involved in overdose
deaths also presents challenges for understanding the drug overdose crisis (Ruhm, 2017; Warner
et al., 2013).
Some states (e.g., Rhode Island) have made strides in improving the timeliness of reporting
for overdose deaths (Rhode Island Department of Health, 2015). Improved timeliness and
consistency of death certificate data can enable states and local communities to more rapidly
identify and respond to overdose spikes, facilitating timelier and more appropriately tailored
interventions (Houry, 2017). Federal programs, such as the CDC’s Data-Driven Prevention
Initiative (CDC, 2017) and Enhanced State Opioid Overdose Surveillance System (CDC, 2017),
are supporting the efforts of states and local authorities to track developments in the opioid crisis
and implement rapid and targeted responses (Price, 2017).
Additionally, better public health surveillance tools for monitoring medical and nonmedical
use of prescription opioids can promote public health and safety. Prescription drug monitoring
programs (PDMPs) are increasingly used to identify opioid analgesic prescribing trends (Katz et
al., 2010; HHS, 2013; O’Kane et al., 2016) and apply risk indicators for inappropriate prescriber
behavior (Ringwalt et al., 2015; Kreiner et al., 2017; Porucznik et al., 2014). Other large
databases, such as all-payers claims databases, are also valuable resources for understanding the
crisis, particularly if they are able to accurately link individuals over time and/or link to other
relevant data sources. However, the usefulness of such systems for analyses requires a data
infrastructure and legal authority for creating linked health databases that are not always
available.
Better Research
Data can be linked at various levels (e.g., individual, county, state, or multilevel linkages);
each approach offers benefits and challenges. Individual-level linkages and analyses are most
5

appropriate for inferring individual-level
relationships (Greenland, 2002; Robinson,
1950; Finney et al., 2011) and longitudinal
data can support analyses of individual-
level prescribing or treatment trajectories
as well as pathways that precede opioid
harms (e.g., overdose) or entry into
treatment. However, very few national data
sources can be linked at the person level,
and efforts to develop such linked data
sources and make them more accessible
must address statistical issues in generating
matches when unique identifiers or full
personal identifiable information are not
universally available across data sets
(Winkler, 2006; Winkler, 1999; Kum et al., 2014; Desetzina et al., 2014; Fellegi and Sunter,
1969). Potential benefits from individual-level analyses must also be balanced with potential
privacy concerns (Doshi et al., 2016; Kho et al., 2015; Ross and Krumholz, 2013). The need for
data owners to maintain protections for individual privacy may also limit the ability to create
person-level linked data files for research. Linking or analyzing data sources at more aggregate
levels is less resource-intensive, but such analyses may be more limited in their potential to
identify many key factors influencing the opioid crisis.
Box 2. General Steps for Conducting Data
Linkages
! Identify the necessary data sets.
! Obtain required approvals from regulatory
authorities, funding sources, and
institutional review boards.
! Select the data elements that will be used to
link across data sources.
! Determine the most appropriate method and
matching algorithms for linking.
! If a gold standard validation method is
available, assess match quality through
metrics such as sensitivity, specificity,
positive and negative predictive value.
The general steps for conducting data linkages are outlined in Box 2 (Bradley et al., 2010;
Dusetzina, Tyree, and Meyer, 2014; Dusetzina et al., 2014). Each step poses potential challenges,
and the most pronounced challenges generally arise in linking data at the individual level. These
include several institutional challenges for obtaining required data approvals. Linking and
obtaining approvals to use data sources hosted by different agencies, which may differ in their
legal obligations, interests, and resource capacities, can be burdensome, time-intensive, and
costly. Even when approval is obtained, there can be substantial statistical challenges in
conducting the linkages, exacerbated in data sets that lack common data elements. Choices must
be made regarding how to define unique person identifiers and to determine the best method(s)
for linking (e.g., deterministic or probabilistic matching, Bayesian approaches, or machine-
learning techniques; see Dusetzina et al. [2014] for a recent overview); and these choices will
influence the quality of matches (Campbell et al., 2008; Clark, 2004; Méray et al., 2007; Sayers
et al., 2016; Asnsolabehere and Hersh, 2017). Errors that may occur during this process, such as
errors of incorrectly linking records that do not belong to the same person (false positive) and
errors of incorrectly failing to link records that belong to the same person (false negative)
influence the rigor of subsequent analyses (Méray et al., 2007; Tromp et al., 2011).
6
In the following sections, we document the more-common types of data and linkages that
researchers are using to advance our understanding of the opioid crisis.
7

3. Current State of the Evidence: Findings from the Environmental
Scan
To gather information about data sets currently used in empirical studies, we conducted an
environmental scan, with special focus on research relevant to the HHS Strategic Priorities. We
also sought to identify common ways in which these data sources are being linked in existing
research.
We searched the peer-reviewed and grey literature for relevant articles, complemented by a
snowball approach, in which we reviewed citations and references in the articles we identified to
identify additional relevant materials that may not have been captured in the original search. As
part of this initial stage of work, we also conducted telephone conversations with five opioid
researchers currently using secondary data sources, several of whom also participated in the
stakeholder discussions described in Chapter 5, to ensure that the literature review did not miss
key data sources. These conversations confirmed the use of data sources identified in the
literature scan but did not identify any additional data sources. In total, we identified 278
documents that we reviewed for the scan, of which 250 were peer-reviewed publications; the
remainder were largely reports, working papers, and newspaper or internet articles.
Below, we summarize the environmental scan’s main findings, grouping research topics,
variables, and data sources by HHS Strategic Priority. The discussion focuses on highlighting
more-common research questions evaluated in the existing literature, as well as the more-
common specific secondary data sources and measures used to answer such questions. Chapter 4
categorizes the types of secondary data sources used in research related to HHS Strategic
Priorities, with more general discussion of differences across data source types. Other important
but less commonly used data sources are described in Chapter 5.
Better Practices for Pain Management
Research has improved the understanding of opioid analgesic prescribing patterns,
prescription fill behavior, and prescription characteristics predictive of misuse or opioid-related
harms. Research has also improved the understanding of the effectiveness of states’ efforts to
advance better pain management practices. PDMPs are the most commonly studied state
initiatives, with more limited research examining the effects of laws-regulating “pill mills,” (i.e.,
clinics prescribing high volumes of opioids with limited clinical oversight), abuse-deterrent
opioid formulations, pain management education, and prescribing guidelines. Table 3.1 lists data
sources and measures commonly used in research related to pain management practices
identified through the environmental scan.
8

The measures identified in Table 3.1 can be used to evaluate how PDMP implementation
affects opioid-related consequences. The measures can also be used to evaluate the trends in
opioid analgesic prescribing and associations with risky prescribing or opioid-related harms.
!"#$%&'()(&*+,,+-$.&/0%1&2"3"&4+567%0&"-1&8%"056%0&9-&:%0%"67;&3+&<1="-7%&>%33%6&?"9-&
8"-"@%,%-3&?6"7397%0&
Data Type Commonly Used Sources Commonly Used Measures
Commercial
insurance
claims
&
• IQVIA
• Marketscan
• Health Care Cost Institute
• Opioid analgesic prescriptions
• Prescribing patterns or prescription-fill behavior
indicative of misuse
• Morphine equivalent daily dose (MEDD)
• Payment type (e.g., Medicare Part D, cash)
Medicaid
claims
• Medicaid State Drug
Utilization file
• State Medicaid data
sources
• Opioid analgesic prescriptions
• Prescribing patterns or prescription-fill behavior
indicative of misuse
• MEDD
• Diagnostic codes for nonfatal overdose
• Payment type
Medicare
claims
• Medicare Prescription Drug
Event data linked to
Medicare Beneficiary
Summary File
• Opioid analgesic prescriptions
• Prescribing patterns or prescription-fill behavior
indicative of misuse
• MEDD
• Diagnostic codes for nonfatal overdose
• Payment type
Electronic
health
records
(EHRs) and
claims data
• National or regional
Veterans Health
Administration (VHA) data
warehouses
• Opioid analgesic prescriptions
• MEDD
• Indicators of prescription opioid abuse or dependence
• Clinical diagnoses (e.g., pain conditions)
PDMP data
• State PDMPs • Opioid analgesic prescriptions
• MEDD
• Prescribing patterns or prescription-fill behavior
indicative of misuse
Mortality data
• National Death Index (NDI)
• National Vital Statistics
System Multiple Cause of
Death (NVSS MCOD)
• CDC WONDER
• State death certificate data
• Opioid overdose fatality
• Injury intent (e.g., suicide, accidental)
Policy data
• Prescription Drug Abuse
Policy System (PDAPS)
• National Alliance for Model
State Drug Laws
(NAMSDL)
•
PDMP enactment
• PDMP design features
Several common research questions can be addressed using a single data source. For
example, research evaluating time trends or geographic variation in opioid analgesic prescribing
9

A
among the general population has used information from state-specific PDMPs or from
commercial insurance claims such as IQVIA. Other studies have assessed prescribing practices
within the Medicaid, Medicare, or veteran populations using administrative claims or EHR data
sets specific to those populations. Five opioid-related indicators and their respective algorithms
developed by CMS for researchers to use with Medicaid and Medicare administrative claims
data were recently made available for public comment (CMS, 2018); these indicators are planned
for inclusion in the CMS Chronic Conditions Data Warehouse.
However, other research questions rely on linked data sets. Research evaluating the effects of
PDMP implementation on opioid-related consequences commonly merges state-level policy data
with state- or county-level data on opioid prescription claims or rates of fatal opioid overdose
from the NVSS MCOD microdata, CDC WONDER, or state-specific death certificate data.
These analyses also generally control for state- or county-level factors linked from other data
sources, such as those noted in Table 3.2. The commonly used state- or county-level measures in
Table 3.2 can be linked with data on opioid-related consequences and state policy data to control
for potential time-varying community-level confounders correlated with opioid outcomes of
interest. These measures can also be used to estimate how community-level factors relate to
opioid analgesic use and associated harms. Community-level factors of interest generally include
socioeconomic factors (e.g., unemployment rate), demographics (e.g., percentage population
male), or measures of health care infrastructure (e.g., physicians per capita).
!"#$%&'(B(&*+-3%C35"$&2"3"&4+567%0&"-1&8%"056%0&*+,,+-$.&D9-E%1&3+&FG9+91&F537+,%&2"3"&9-&
:%0%"67;&:%$"3%1&3+&3;%&H9=%
?+9-3&II4&436"3%@.&
Data Sources Commonly Used Measures
Bureau of Economic Analysis
• Unemployment rate
• Per capita income
Area Resource Files or Health
Resources Files
• Unemployment rate, per capita income, urban-rural status
• Demographics (e.g., age, sex, race/ethnicity distribution)
• Number of hospital beds per capita, physician density
American Community Survey
• Poverty rates, unemployment rate, education distribution
• Median home prices, median age of housing stock
• Demographics (e.g., age, sex, race/ethnicity distribution)
• Rates of public and private health insurance coverage
Current Population Survey
• Rates of health insurance coverage
• Demographics (e.g., age, sex, race/ethnicity, marital status)
•
Unemployment rate; poverty rates
CMS
• Rates of Medicaid and/or Medicare coverage
Studies evaluating the association of opioid analgesic prescribing patterns or prescription-fill
behavior with opioid-related harms often require data sources linked at the individual level.
10
Noted data-linkage strategies include linking state-specific PDMP data with other data sources,
such as Medicaid administrative claims, hospital discharge data, or vital records; using multiple
linked VHA databases, which have also been linked at the individual level to mortality data from
the NDI; linking Medicaid claims with state vital records data; and using Medicare Prescription
Drug Event data linked with the Medicare Beneficiary Summary file. While not commonly used
in existing opioid-related research, information from the Medicare Current Beneficiary Survey, a
survey of a nationally representative sample of Medicare beneficiaries released three times
annually, has been linked at the patient-level to Medicare billing claims (Wright et al., 2014).
Better Addiction Prevention, Treatment, and Recovery Services
Researchers commonly evaluate how policies intended to expand the number of waivered
buprenorphine prescribers (i.e., prescribers who have received a waiver from the Drug
Enforcement Agency (DEA) allowing them to prescribe buprenorphine for the treatment of
opioid use disorder) relate to buprenorphine prescribing, factors that predict the availability of
waivered prescribers, and factors associated with the monthly patient censuses of waivered
prescribers. Some studies investigate patterns of buprenorphine use among those receiving
opioid use disorder treatment. Data sources and measures commonly used in research related to
opioid use disorder and treatment are shown in Table 3.3.
The measures in Table 3.3 may be used to evaluate trends and geographic variation in
treatment need and opioid agonist treatment capacity, as well as associations between individual-
level characteristics, opioid analgesic use, and opioid use disorder. They can also be used to
evaluate trends, geographic variation, and factors associated with buprenorphine physician
supply. Lastly, they can be used to evaluate national trends and patient trajectories in treatment
for opioid use disorder.
11

!"#$%&'('(&*+,,+-$.&/0%1&2"3"&4+567%0&"-1&8%"056%0&9-&:%0%"67;&3+&J,G6+=%&<119739+-&
?6%=%-39+-K&!6%"3,%-3K&"-1&:%7+=%6.&4%6=97%0&&
Data Type Commonly Used Sources& Commonly Used Measures
Commercial
insurance
claims
• IQVIA&
• Symphony Health
• Buprenorphine prescriptions
• Patient censuses of buprenorphine prescribers
PDMP data
• State-specific PDMPs • Buprenorphine prescriptions
• Patient censuses of buprenorphine prescribers
Medicaid
claims
• National or state Medicaid
data sources
• Buprenorphine prescriptions
• Patient censuses of buprenorphine prescribers
• Opioid use disorder diagnoses
EHR
• HealthCore Integrated
Research Database&
• Group Health Cooperative
• National or regional VHA
data warehouses
• Prescription opioid abuse or dependence
• Diagnostic measures of pain
• Opioid analgesic prescriptions
• Other clinical diagnoses, comorbidities, demographic
characteristics
Household
surveys
• National Survey on Drug
Use and Health (NSDUH)&
• National Epidemiologic
Survey on Alcohol and
Related Conditions
(NESARC)
• Opioid use disorder treatment need
• Treatment source or source of payment
• Opioid use disorder
• Nonmedical prescription opioid misuse
• Other substance use disorders, mental health
conditions, and demographic characteristics
Treatment
facility
surveys
• Treatment Episodes Data
Set-Admissions (TEDS-A)&
• National Survey of
Substance Abuse
Treatment Services (N-
SSATS)
• Number of patients receiving methadone in opioid
treatment programs (OTPs)
• Outpatient operating capacity of OTPs
• Number of substance abuse treatment programs
providing methadone and/or buprenorphine
• Substance abuse treatment services offered
• Number of t
reatment admissions for opioid use disorder
Provider
census
• Substance Abuse and
Mental Health Services
Administration (SAMHSA)
database&
• DEA Active Controlled
Substances Act
Registrants Database
(ACSA)
• Number of buprenorphine providers
• Waiver limits
• Buprenorphine treatment capacity
Policy data
• RAND/National
Conference of State
Legislators Survey
• State Medicaid reimbursement policies for
buprenorphine
Research studying associations between individual-level characteristics, opioid analgesic use,
and opioid use disorder leverages data sources that contain person-level information on these
measures within the same data set. Relevant data sources include household surveys such as the
NSDUH series managed by SAMHSA, NESARC sponsored by the National Institute on Alcohol
12
Abuse and Alcoholism, as well as EHR and claims data from various sources (Table 3.3).
Research examining trends or geographic variation in demand or capacity for opioid use disorder
treatment instead often uses measures from treatment facility surveys, such as the TEDS-A or N-
SSATS, both of which are maintained by SAMHSA.
While studies assessing trends or geographic variation in treatment need and treatment
capacity may advance research using measures from a single data source, a more comprehensive
picture of the relationship between demand for and supply of treatment has been obtained by
linking data sources. For example, studies estimating treatment shortage areas commonly merge
information on treatment need with information on treatment capacity at the state- or county-
level.
Researchers have also used data linkages to better understand factors associated with
buprenorphine prescriber supply and buprenorphine utilization. Information on buprenorphine
prescriber locations is available through two commonly used sources: SAMHSA’s
Buprenorphine Waiver Notification System or the Drug Enforcement Agency Active Controlled
Substances Act Registrants database (DEA ACSA). Information on buprenorphine prescriptions
often comes from insurance claims data or PDMP data. By linking information on buprenorphine
prescribers or prescriptions with state-level policy and county-level contextual factors relevant
for opioid use disorder treatment, research can improve the understanding of factors associated
with buprenorphine treatment capacity and utilization.
Better Targeting of Overdose-Reversing Drugs
The most commonly studied interventions promoting use of overdose reversing drugs are
community-based overdose education and naloxone distribution (OEND) programs. Emerging
evidence focuses on state laws intended to increase naloxone access through retail pharmacy
distribution channels (Naloxone Access Laws) or to encourage community bystanders to
summon emergency aid or administer naloxone in the event of witnessing an overdose (Good
Samaritan Laws). Table 3.4 lists the most commonly used variables and secondary data sources
identified in research related to overdose-reversing drugs.
The measures noted in Table 3.4 can be used to evaluate trends or geographic variation in the
distribution of naloxone through retail pharmacies, presence of community-based OEND
programs, and naloxone administrations by emergency medical services (EMS) personnel. They
can also be used to study how state naloxone policies influence opioid overdose mortality or the
role of OEND programs in impacting knowledge about how to respond to a witnessed overdose,
distribution of naloxone kits and naloxone administrations, and overdose reversals.
13

!"#$%&'(L(&*+,,+-$.&/0%1&2"3"&4+567%0&"-1&8%"056%0&9-&:%0%"67;&3+&J-M+6,&>%33%6&!"6@%39-@&+M&
F=%61+0%-:%=%609-@&265@0&
Data Type Commonly used sources Commonly used measures
Commercial
insurance claims
• IQVIA • Naloxone prescriptions through retail
pharmacy channels
• Prescriber specialty
• Patient age, gender
Mortality data
• CDC WONDER
• NVSS MCOD
• Opioid analgesic overdose deaths
• Heroin overdose deaths
• Synthetic opioid overdose deaths
OEND program data
• Massachusetts Opioid
Overdose Prevention Pilot
Program
• Harm Reduction Coalition
• Reported overdose reversals
• Number of naloxone administrations
• Number persons trained and naloxone kits
distributed
• Knowledge about how to respond to a
witnessed overdose and administer naloxone
EMS data
• NEMSIS
• EMS naloxone administration
Policy data
• PDAPS
• Network of Public Health
Law (NPHL)
• Legal databases
• Good Samaritan laws
• Naloxone access laws
Research on policies or programs to expand naloxone use often rely on data from a single
source. Studies of the effects of community-based OEND programs on overdose knowledge and
outcomes generally rely on case studies using surveys of OEND program participants or other
data collected by the specific OEND programs. Other research has documented the evolution of
state laws governing naloxone access and use, drawing on review of legal databases to obtain
information about state policies related to naloxone access and use for community bystanders or
first responders. Finally, some studies have described trends in naloxone distribution through
different channels using retail pharmacy naloxone distribution (IQVIA) or EMS naloxone
administration (National Emergency Medical Services Information System [NEMSIS]).
Data linkages are most commonly used to examine the effects of state naloxone policies or
OEND programs on opioid overdose. Such research commonly merges state- or county-level
mortality data from the NVSS MCOD microdata or CDC WONDER with state-level information
on naloxone access policies or Good Samaritan Laws compiled by the Prescription Drug Abuse
Policy System (PDAPS) or the NPHL program. Studies of state naloxone policy effects also
commonly control for other state- or county-level contextual factors as described in Table 3.2.
Other state-specific analyses use multiple complementary data sources to examine whether
implementation of a community OEND program (Albert et al., 2011) influences trends in
emergency department visits for substance abuse and accidental poisonings, opioid overdose
mortality, and outpatient-dispensed controlled substances.
14
Better Data
Researchers concerned with surveillance often use multiple complementary data sources to
better understand trends and disparities related to the opioid crisis, develop methods to improve
monitoring through existing public health surveillance systems (e.g., EHR, emergency
department encounter data), identify patients at high risk of prescription opioid misuse or abuse,
and promote improved opioid toxicosurveillance (i.e., rapid analysis of drug exposure data).
Below we briefly describe the data sources and measures most commonly used to strengthen
public health surveillance research.
Much public health surveillance research uses near-real time surveillance tools to better
understand product-specific abuse and emerging trends. Three databases have been designed to
provide near-real-time surveillance data on opioid misuse: the Researched Abuse, Diversion and
Addiction-Related Surveillance System (RADARS), the National Addictions Vigilance
Intervention and Prevention Program (NAVIPPRO), and the Prescription Behavior Surveillance
System (PBSS). The RADARS and NAVIPPRO compile information on opioid use,
consequences, and markets from multiple sources; the PBSS compiles state-specific PDMP
information from several states. In addition, opioid overdose information collected from poison
control centers through the National Poison Data System (NPDS) has been used by research and
surveillance efforts to capture product-specific opioid overdose events that may not result in
death.
Data costs or other barriers to access may limit widespread use of these systems in existing
research; however, they are increasingly used in studies related to problematic opioid use and
product-specific abuse trends. Data collected through online social media has also been
increasingly used to monitor illicit or problem opioid use (Parker et al., 2017; Katsuki et al.,
2015; Anderson et al., 2017).
Significant progress has been made in developing metrics and leveraging existing
surveillance systems to better detect opioid misuse or potentially inappropriate prescribing. As
detailed in the prior sections, information on opioid prescriptions and opioid misuse indicators
are available through multiple data sources, including claims and EHR data. State-specific
PDMP data and all-payers claims databases (APCDs) are also emerging as useful data sources to
better understand opioid prescribing and potential misuse. While we identified fewer studies
examining illicit opioids, some studies have used local law enforcement data on drug seizures or
arrests to better understand heroin markets, illicit opioid analgesic markets, and illicit markets for
synthetic opioids. Other research using RADARS, NAVIPPRO, and the NSDUH has examined
sources of prescription opioids and measures of prescription opioid diversion.
A common data-linking strategy for public health surveillance is to leverage multiple data
sets and conduct complementary analyses of state- or county-level information to better
understand the evolution of the opioid crisis. For example, studies have linked individual-level
15
prescription data from PDMPs or Medicaid claims with state death certificate data to examine
trends in prescribing behavior preceding overdose death.
States are also implementing strategies to better link and analyze data across state agencies.
For example, with Chapter 55 of the Acts of 2015, Massachusetts’ Department of Public Health
has connected ten data sources managed by five state agencies to develop a data warehouse
structure. These data sources include the state APCD; the Massachusetts PDMP; death certificate
records and toxicology results; substance abuse treatment information; hospital, emergency
department, and outpatient records; incarceration and criminal justice system treatment records;
and emergency medical service incident data from licensed ambulance services. Chapter 55 is
discussed further in Chapter 6 of this report.
16

4. Sources of Secondary Data: Data Inventory Findings
In Chapter 3, we provided an overview of
the more commonly identified research
questions that secondary data sources have
been used to examine, organized by HHS
Strategic Priorities. However, our
environmental scan uncovered a broader array
of existing data resources relevant to the HHS
Strategic Priorities. In Table 4.1, we
categorize and describe the types of additional
secondary data sources and provide examples
of common data sources and variables within each type.
Box 3. Major Sources of Secondary Data
! National surveys
! Claims and EHR data sources
! Mortality record data sources
! Prescription drug monitoring data
sources
! Contextual and policy data sources
! Other national, state, or local data
sources
Box 3 highlights the six broad sources of data we identified: (1) national surveys, (2) EHR
and claims data, (3) mortality records, (4) prescription drug-monitoring data, (5) contextual and
policy data, and (6) other national, state, or local data sources (e.g., national poison control
center data, state arrest records). The full data inventory provided in the appendix to this report
contains more-detailed information on each identified data set within these broader categories.
This information includes the agency hosting the data and type of data; a high-level summary of
data content, including geographic coverage, timing of collection or data availability, and
important measures; information on accessing the data, including a link to the website,
information on access costs, and other restrictions; a link to any available analytics; and
information on linking capability.
17
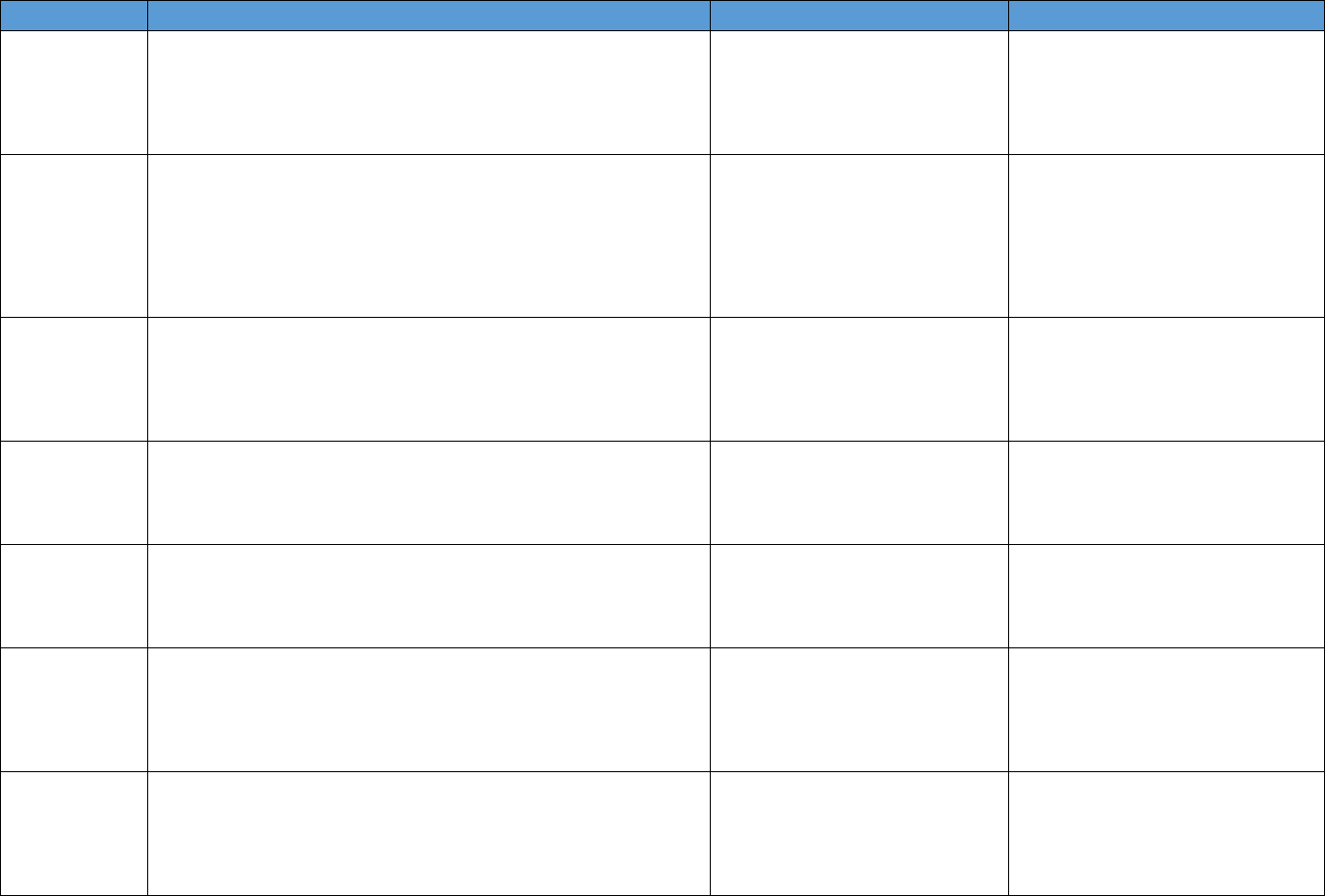
Table 4.1. Data Source Categories Identified
Data Description Summary Examples of Important Measures Data Source Examples
National surveys
Description: Generally household or school4based surveys with self4
reported information on drug use and health; other surveys are of
hospitals, treatment facilities, or other medical service providers
Geographic coverage: National
Timing: Generally collected and available annually
Prescription opioid use, heroin use,
opioid use disorder, medical
conditions, health care utilization
National Survey on Drug Use and
Health, National Ambulatory Medical
Care Survey, National Survey of
Substance Abuse Treatment Services
Data, Medical Expenditure Panel
Survey
EHR Description: An EHR contains the medical and treatment histories of
patients. However, it often contains more than standard clinical data,
and may also include a broader view of a patient’s care. EHRs may
contain a
patient’s medical history, diagnoses, medications, treatment
plans, allergies, radiology images, and laboratory and test results
Geographic coverage: Varies by source
Timing: Near4real time or real4time collection
Previously prescribed opioids or
other medications; patient history,
medications, clinical conditions,
treatment plans, and lab/test
results; may include clinician notes
Stanford Translational Research
Integrated Database, HealthCore
Integrated Research Database, Group
Health Cooperative in Washington
State
Claims data
Description: Patient4level claims data for reimbursement for services
submitted by health care providers and pharmacies to insurance
companies. Validated algorithms to identify opioid misuse or abuse
from claims data are being developed
Geographic coverage: Varies by source
Timing: Varies by source
Prescription drug utilization; service
utilization
IQVIA, Symphony Health, Truven
Marketscan data, Medicaid claims,
Medicare Part D Prescription Drug
Event data
Mortality records
Description: Death rates and causes of death by drug compound
and/or International Classification of Diseases code. Additional
information can include toxicology reports
Geographic coverage: National or single state
Timing: Generally available annually
Rates of opioid4involved deaths;
drugs involved in overdose deaths
CDC WONDER Multiple4cause4of death
data; Fatal Accident Reporting System;
NDI
Prescription
monitoring data
Description: Data systems to track and monitor the distribution or
prescription of controlled substances
Geographic coverage: Varies by source
Timing: Varies by source
Opioid prescribing rates (by type);
indicators of "doctor shopping,"
coprescribing of opioids and other
controlled drugs, geographic
variation in opioid distribution
Automation of Reports and
Consolidated Orders System (ARCOS);
state prescription drug–monitoring
programs
Contextual and
policy data
Description: Causal analyses of the effects of policy changes on
opioid4related outcomes generally use data on state laws from these
sources and/or includes controls for state or county characteristics to
support causal interpretation
Geographic coverage: National
Timing: Varies, but generally semiannually
State opioid policies, state and
county demographic and
socioeconomic factors, state and
county health care variables
Area Health Resources Files, Policy
Surveillance System, PDAPS
Other national,
state, and local
sources
Description: Includes data collected through law enforcement,
national public health surveillance systems (e.g., poison control center
data, emergency department visit data), OEND program data, other
hospitalization and emergency department data
Geographic coverage: Varies by source
Timing: Varies by source
Law enforcement drug seizures,
nonfatal opioid overdose, opioid4
related emergency department visits
and hospitalizations, naloxone
distribution through community
organizations
NEMSIS, NPDS, HCUP emergency
department and hospitalization data
18
National Surveys
National survey data sources, often collected annually, include population-based surveys,
such as household surveys or school-based surveys, as well as surveys of medical providers,
hospitals, emergency departments, and treatment facilities. Population-based surveys often
include self-reported information on lifetime or current heroin or opioid analgesic use, symptoms
of opioid use disorder, and treatment or unmet treatment need for opioid use disorder; as well as
a variety of measures describing respondent demographics, socioeconomics, and other mental
health or substance use behaviors. Systematic data collection over time supports trend analyses at
the national and sometimes state or local level; however, significant changes to survey design or
implementation may limit longitudinal comparisons.
One caveat with regard to many national population-based surveys is that they restrict their
sample to the civilian, noninstitutionalized population, thus excluding some high-risk groups,
such as homeless individuals not residing in shelters and incarcerated individuals. However, a
few national surveys, such as the Arrestee Drug Abuse Monitoring System (ADAM) and the
National HIV Behavioral Surveillance System, have focused specifically on high-risk
populations, arrestees, and persons at risk for HIV infection.
Other national survey data-collection efforts gather information from hospitals, emergency
departments, and outpatient departments. These data sources offer information on prescriptions
received through various health care settings as well as acute health care visits attributable to
opioid use or misuse; data from three of these surveys have been integrated into the National
Hospital Care Survey (CDC, 2015). Finally, national surveys of mental health or substance abuse
treatment facilities collect information relevant to treatment utilization and treatment capacity for
opioid use disorder.
While most national survey data sources (with some exceptions, see Table A.1 in the
appendix) allow public access at no cost, access to certain data elements may be restricted.
Restricted data elements often include geocoded variables that would allow analyses or linkages
at the state or substate level. Obtaining access to these geocoded variables typically involves an
application process; use of such information is often only allowed through a Research Data
Center (U.S. Census Bureau, 2015) or other secure access data portal and, in some cases, is
restricted to use by federal employees. Similarly, while several national surveys permit person-
level linkages with other national data sources (e.g., the National Health Interview Survey [CDC,
2017] supports person-level linkages with the NDI, Medicare data sources, and AHRQ’s Medical
Expenditure Panel Survey) upon approval of the research project, access to the linked files is
typically only permitted through secure Research Data Centers. Currently, national survey data
from substance use treatment facilities may not be linked to units below the county level.
19
Electronic Health Records and Claims Data
An EHR is an electronic version of a patient’s medical history. It may include a variety of
key clinical data, including demographics, medical history, medications, progress notes,
problems, and other physician or nurse documentation. Efforts to expand the adoption and use of
EHRs have been focused primarily on improving the quality of health care (Appari et al., 2013;
Blumenthal and Tavenner, 2010; Campanella et al., 2016). However, there has been growing
interest in using EHR data for public health surveillance and response efforts (Friedman, Parrish,
and Ross, 2013; Coorevits et al., 2013). EHRs have been proposed as a tool to help practitioners
implement better pain assessment and management practices (Anderson et al., 2016; Harle et al.,
2014), as well as a potential data resource to better identify factors associated with opioid
misuse, adverse events, or development of opioid use disorder (Lingren et al, 2018; Hser et al.,
2017; Green et al., 2017; Carrell et al., 2017). Typically available in real time, EHR systems may
contain a variety of measures, such as health behaviors indicative of opioid misuse, that may not
be needed for billing purposes and thus would not be captured in claims data. For example,
EHRs may contain relevant laboratory values, such as urine drug screens, as well as allowing a
calculation of abandoned opioid analgesic prescriptions (prescriptions that are written but never
filled by patients).
However, there are several challenges to using EHR data, including issues with fragmented
or incomplete data, the need for text note processing and validation, and a lack of consistency in
methods to assess EHR data quality (Madden et al., 2016; Weiskopf and Weng, 2013; Häyrinen,
Saranto, and Nykänen, 2008; Raghupathi and Raghupathi, 2014). Data-quality concerns can
generate serious issues in determining unique patient identifiers, which in turn creates errors in
person-level record linkage with other data sources (McCoy et al., 2013; Murray, 2014).
Challenges with gaining approvals and access to EHR data may also restrict the use of EHR data
in secondary research (Russo et al., 2016).
Table 4.2 compares EHR and administrative claims data sources. Because claims data are
intended to support reimbursement for services submitted by health care providers and
pharmacies to insurance companies, they tend to have fewer data-quality issues, have a more-
standardized structure and method for entering data, and assign standardized definitions for data-
point entry. Claims records can come from data sources hosted by a single federal insurer, single
state insurer, integrated database of a privately insured population, multipayer claims database
owned by a private agency, or state all-payer claims database. While access restrictions are often
not as burdensome as those for EHR data, the required approval process and costs of obtaining
person-level claims data may be a barrier to use for research purposes.
20
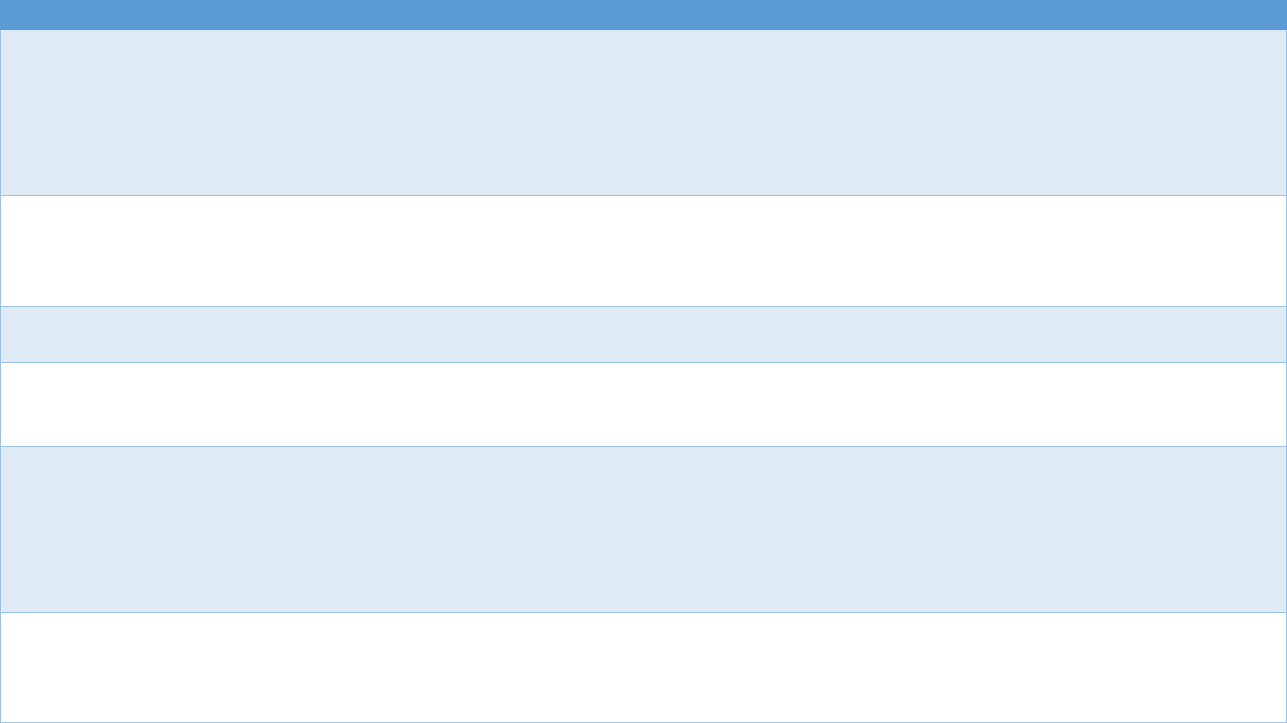
Table 4.2. Comparison of Electronic Health Record and Administrative Claims Data
EHR Data Insurance Claims Data
Coverage or source of data set
(examples)
● Single institution (private)
● Health information exchanges or group health
network
● National or regional VHA systems
● Commercial claims from private payers
● Federal and state claims (Medicaid, Medicare)
● Integrated databases with medical and pharmacy
claims
Potential scope of patients All patients, including those with no insurance
coverage (in systems that have adopted an
EHR)
Insured patients, may be restricted to single payer
population
Breadth of data Richer data but greater variability in data
element availability
More limited set of data elements but more
standardized collection
Prescription data Information on whether medication was
prescribed, not whether it was filled or refilled
Detailed information on filled prescriptions and
refilled prescriptions (assuming there was a claim)
Data structure and quality Data format, completeness, and overall quality
can vary greatly. Researcher may need to
operationalize how variables of interest are
defined, and this may look different with different
EHRs
Fairly standardized claim data formats, although data
warehouse structures can vary by payer. Variables
(e.g., diagnostic codes, drug dispensing) typically
well-defined and complete when required for
payment
Data access May require on-site access, remote access may
be restricted to limited data set, security
protocols, costs unclear
Costs vary depending on request. Some data must
be requested and approved. Varying privacy levels
for some CMS Medicaid and Medicare files
21
Mortality Records
Information on opioid overdose mortality from death records and postmortem toxicology
data can be obtained from state-specific death certificate or from national data sets that compile
death certificate data submitted by states into a single source. There are three primary sources for
national data on mortality, one of which (CDC WONDER [CDC, 2018]) is publicly available,
does not carry fees, and supports readily downloadable data files through an easy-to-use online
system. However, the public version of the multiple-cause-of-death files provided through CDC
WONDER masks subnational estimates in which fewer than ten deaths occurred. Thus, for
county- or state-level analyses stratified by demographic variables—where cell sizes may
become quite small—obtaining access to the underlying NVSS MCOD microdata may be
necessary (national opioid mortality data analytics are available online [CDC, 2017]). While one
limitation of mortality data is the long lag time for data to become available, the Vital Statistics
Rapid Release Provision Drug Overdose Death Counts (CDC, 2018) is an effort by the National
Center for Health Statistics to provide timelier information on drug overdose mortality based on
provisional fatality counts from the NVSS MCOD.
While both CDC WONDER and NVSS MCOD support linkages and county-level analyses,
person-level linkages with national geographic coverage are only supported through the NDI, a
centralized national database of death records that is not available to the general public, has a fee
schedule with charges per record requested, and entails costs to obtain cause-of-death
information. The NDI can be linked at the individual level to multiple other data sources,
including national surveys, VHA health care data, and other national or state sources. State death
records, while not publicly available, can also be linked at the person level to other state-specific
databases, including PDMP data.
Prescription Drug–Monitoring Data
Prescription drug–monitoring data sources are those designed to monitor controlled
substance prescribing, distribution, or dispensation. These include a federal database monitoring
national distribution of controlled substances from manufacture to sale (i.e., ARCOS) as well as
state PDMP systems, electronic databases generally hosted by a state licensing, health, or
criminal justice agency and intended to track controlled prescription drugs dispensed to patients
within the state (Pardo, 2017). The lag time for data reporting, degree of coverage, ability to
identify providers, and specific measures captured within a given PDMP system vary across
states depending on the state law regulating the PDMP (Greenwood-Ericksen et al., 2016;
Manasco et al., 2016).
States also vary in the degree to which their state PDMP system allows interstate information
sharing, authorizes access for research and public health purposes, and/or permits person-level
linkage to other state-owned data sources. As of December 5, 2017, 48 states and U.S. territories
22
are authorized to provide de-identified PDMP data to researchers, and 25 of these states have
released PDMP data for research, epidemiological, or educational purposes (PDMP, 2017).
Contextual and Policy Data
Contextual data sources are generally used in opioid research to assess state- or county-level
factors associated with opioid-related outcomes or to account for time-varying state- or county-
level demographic, health care, or socioeconomic factors that may confound estimation in
analyses of policies targeting opioid use, treatment, or opioid-related harms. When used in
research related to the HHS strategic areas, measures derived from contextual data sources are
generally obtained at more aggregate levels (e.g., state, county) or are aggregated up from
person-level data sources to the state or county level.
Most contextual data sources are hosted by federal agencies, although some private
organizations (e.g., Kaiser Family Foundation) and some federal entities (e.g., the Health
Resources and Services Administration) compile information from several federally hosted
contextual data sources into a single location and also maintain their own data sources.
Depending on the source, data may be representative at the state or substate level, with supported
linkage or unit of analysis as finely geographically detailed as the ZIP level (e.g., the U.S.
Census Bureau Zip Code Business Patterns data) (Cerdá et al,. 2017), although this level of detail
is generally not available in public data sets. Additionally, contextual information compiled from
national person-level survey data sources (e.g., the Current Population Survey) is less likely to be
representative at the substate level (Blewett and Davern, 2006) or to provide microdata for all
counties. Reviewing all contextual data sources identified through the environmental scan was
outside the scope of this project. However, we highlight a few of the most commonly used data
sources in Table A.5 in the appendix.
Policy data sources capture information on state opioid policies and thus are generally
analyzed and linked using state as the unit of analysis. A variety of agencies, including federal,
federally funded, and private organizations, collect information on state opioid policies.
Information on state PDMP policies, naloxone access laws, and Good Samaritan laws have been
compiled by several sources, including PDAPS and NAMSDL, although these sources often vary
in the exact classification they use to define the components and timing of such laws. In many
cases, policy data are publicly available at no cost. However, free and publicly available policy
data are often not provided in analytic formats or as a historical data set; instead, they often
represent a “snapshot” of current policies. Additionally, few data sources are available that
systematically track and provide information on how state opioid policies are being
implemented, note changes in local efforts related to the opioid crisis (e.g., law enforcement
carrying naloxone), or describe large-scale opioid policies or guidelines implemented by payers
or health care systems to address opioid prescribing.
23
Other National, State, and Local Sources
Several data sources relevant to the HHS opioid strategies do not directly fit within any of the
aforementioned categories. These include national censuses of waivered buprenorphine
providers; national proprietary data systems, such as RADARS, that combine information from
various sources to describe and surveil misuse, abuse, and diversion of prescription drugs; and
national data on emergency medical services utilization such as NEMSIS, drugs seized by law
enforcement, and calls to poison control centers.
This data source category also includes a suite of national- and state-level data products
capturing hospital inpatient stays and hospital-based emergency department visits available
through the Healthcare Cost and Utilization Project (HCUP), managed by the Agency for
Healthcare Research and Quality (AHRQ). Access to the state or national HCUP data files must
be applied for and purchased; however, the HCUP website offers a publicly available online
query system (Agency for Healthcare Research and Quality, 2018) and a limited set of user-
friendly graphics and tables showing state and national trends in opioid-related inpatient stays
and emergency department visits (Healthcare Cost and Utilization Project, 2018). Finally,
increased public attention to the opioid crisis has led to the emergence of online state opioid
dashboards; new opioid data-compilation efforts; as well as increased attention to data sources
that may capture the complex role of clinical conditions, health care delivery and access,
prescribing, and opioid misuse or development of opioid use disorder (see Box 4 for examples).
24

Box 4. Other Data Sources Relevant to the HHS Strategic Priorities
The data inventory was intended to provide an overview of commonly used secondary
data sources in research related to the HHS strategic areas. It is not an exhaustive list of
secondary data currently or potentially available to further our understanding of the opioid
crisis. We here note several data sources that are not commonly used in existing research, but
may be of interest.
! State opioid dashboards provide state statistics related to the opioid crisis. Examples
include
• Arizona Department of Health Service’s Arizona’s Real-Time Opioid Data
(2017–2018)
• Minnesorta Department of Public Health’s Opioid Dashboard (undated)
• Tennessee Department of Health’s Drug Overdose Dashboard (undated)
! National opioid data collections compile or support the compilation of relevant data
from a variety of sources into a single location. Examples include
• Opioid and Health Indicators Database by amfAR (undated), the Foundation
for AIDS Research
• Opioid Mapping Initiative (undated), an open-data project with several
participating local governments and local agencies
! PCORNet Clinical Data Research Networks include a range of participating health
care–based networks (pcornet, undated) engaged in partnering to link claims and EHR
data. These include resources such as the Chicago Area Patient Centered Outcomes
Research Network (Capricorn, undated) and OCHIN’s Data Warehouse (OCHIN,
2014–2018)
! The Health Resources and Services Administration (HRSA)’s Health Center Program
offers several resources, including
• HRSA’s Uniform Data System (HRSA, 2018) provides publicly available
aggregate data on patients who have opioid use disorder diagnoses or who are
receiving medication-assisted treatment through HRSA-funded health center
grantees and lookalikes.
• The Health Center Patient Survey (HCPS) data, made available with support
from Assistant Secretary for Planning and Evaluation, provides information on
health center patients’ conditions and demographics, health behaviors, service
use, and satisfaction (HRSA, undated).
25

5. High-Priority Research Needs and Data Efforts:
Findings from the Stakeholder Discussions
To assess high-priority research areas and data efforts relevant to the HHS strategy, we
conducted a set of stakeholder discussions to gather insights into opportunities to enhance data
collection and data linkages. In consultation with staff within the Office of the Assistant
Secretary for Planning and Evaluation, we identified 25 key stakeholders with particular
expertise or research experience related to the HHS strategy, 16 of whom participated in phone
discussions. Each discussion was tailored and focused on the HHS strategy about which the
stakeholder was most knowledgeable.
In this section, we highlight themes that emerged from stakeholder discussions of research
opportunities using secondary data sources to support the HHS strategy. We also provide a table
summarizing strengths and limitations of data sources that stakeholders referenced with respect
to each Strategic Priority. The appendix to this report provides additional data source details.
Better Practices for Pain Management
Common themes emerging from discussions related to key research aims for advancing
better practices for pain management include:
• Opioid prescribing guidelines and clinician education: Better documentation of
opioid-prescribing guidelines and clinician education requirements, linked with outcome
data at the prescriber or patient level, would shed light on how variation in these
protocols relates to variation in treatment for pain, and how this in turn impacts patient
outcomes.
• Nonopioid treatments for pain: Opioid analgesics may not be more effective than other
treatments in the management of many tyes of long-term pain (Krebs et al., 2010; Krebs
et al., 2018). More evidence is needed regarding the full range of long-term effective
treatments for chronic pain, including combinations that might be more effective than
opioid analgesics.
• Patient trajectories: Longitudinal patient-level data linking prescriptions with outcomes
can enhance better understanding of the pathways and sequences of events leading to
adverse outcomes such as hospitalization and overdose death. Medicaid and commercial
claims data can be useful, but each provide information on only one population and often
cannot track individuals when they transition across different types of insurance (Table
5.1). APCDs (in states that have them) provide a comprehensive picture of health care
claims across a state’s insured population to track utilization and compare rates across
different populations with different types of insurance, although the ability to track
patients across changes in insurance varies by state (The Commonwealth of
Massachusetts, Executive Office of Human Services, Department of Public Health,
2017).
26
Table 5.1 highlights common data source strengths and limitations noted during stakeholder
discussions regarding better practices for pain management. Key takeaways regarding the
advantages and limitations of various data source types include the following:
• Overall, EHR, PDMP, and claims data can provide detailed information on prescription
characteristics and payment, but the systems may not allow longitudinal follow-up of a
given individual across longer periods of time or across insurance coverage transitions.
• While commercial claims and PDMP data may have strengths in capturing information
from multiple payers, Medicaid claims and VHA data warehouses appear to better
support individual-level linkages with other national-level data sources, such as national
mortality records.
• The ability to conduct cross-state analyses may bolster research examining the effects of
interventions on prescribing outcomes, and the compilation of historical information on
PDMP enactment in several data sources has supported such research.
• Other efforts to target opioid prescribing (e.g., guidelines, prescribing limits) have not yet
been systematically collected in a way that facilitates research on their effects.
27

Table 5.1. Commonly Referenced Data Sources for Understanding Better Practices for Pain Management
Data Type and Example
Sources
Strengths Limitations
Commercial claims
• IQVIA
• Truven
• Multipayer; may include cash payments (e.g., IQVIA)
• Captures detail on opioid analgesic prescription
characteristics and other prescriptions filled
• Data systems are not set up to track people long-
term given insurance coverage transitions
• Limited information on diagnoses or other health
care utilization
• Difficult to link to outcomes (e.g., mortality)
Medicaid claims
• National or state Medicaid
data sources
• Can link hospital and pharmacy claims
• Can look at prescription histories of patients who
make it to the hospital or emergency department for
fatal or nonfatal overdose
• Captures detail on opioid analgesic prescription
characteristics and other prescriptions filled
• Only provides information on one population
(Medicaid enrollees)
• Data systems are not set up to track people long-
term given insurance coverage transitions
• Cannot measure opioid mortality: dates of death
commonly not available and cause of death not
included
EHR and claims data
• National or regional VHA
data warehouses
• VHA data warehouse enables linkages across
multiple VHA data sources
• VHA data have been linked with NDI to connect
prescribing to mortality
• Captures detail on opioid analgesic prescription
characteristics and other prescriptions filled
• Access is highly limited
• Findings from veteran population may not be
directly generalizable to other populations
PDMP data
• State PDMPs
• PBSS
• Not restricted to one payer
• Can be used to develop measures around patient,
prescriber, and pharmacist risky behaviors
• Detail on scheduled substance prescriptions
(coverage varies across states)
• Access barriers
• Many states have capacity issues that limit ability to
link PDMP data with other data sources
• Many PDMPs do not collect unique identiers or
have errors in entry, creating technical issues in
matching at the individual level
28
Data Type and Example
Sources
Strengths Limitations
Mortality data
• NDI
• NVSS MCOD
• CDC WONDER
• State death certificate
data
• Information on cause of death and drugs involved
• NDI has been linked at person-level to other data
sources
• State vital records can offer detail on cause of death
• CDC WONDER publicly available
• Generally updated annually; up to 11-month delay
• Data request and approval can take up to three
months
• For NDI, cause of death codes are an additional
cost
Policy data
• PDAPS
• NAMSDL
• Information on PDMPs, pain clinic laws, education
requirements, prescribing limits
• Can be linked with outcome data to examine impact
of state policies
• Some data not provided in analyzable format
• Some policy information not provided available
historically (e.g., only provides a snapshot)
29
Better Addiction Prevention, Treatment, and Recovery Services
Common themes emerging from discussions related to improving access to treatment and
recovery services include the following:
• Supply of treatment: Understanding how policies and initiatives are influencing access
to treatment and recovery services requires access to treatment supply and capacity data.
Claims data and data on Drug Addiction Treatment Act–waivered physicians have been
used to examine buprenorphine treatment capacity (Table 5.2) (Rosenblatt et al., 2015;
Knudsen et al., 2015; Stein et al., 2015; Stein et al., 2015; Dick et al., 2015). However,
developing a fully comprehensive picture of the treatment landscape is challenging: We
lack data on individuals receiving methadone from opioid treatment programs or
receiving treatment provided under state block grants, federal grants provided to support
substance abuse treatment services that are not tied to public or private insurance.
• Treatment demand and utilization: Better understanding the size and characteristics of
the population with opioid use disorder, and who gets treatment, could inform efforts to
close the treatment gap. Analyses of national cross-sectional surveys and claims data
have been useful, but longitudinal data with unique patient identifiers would allow
longer-term analyses of treatment patterns, identifying gaps or limited access points,
events leading to induction or dropout, and processes to improve continued abstinence.
• Treatment processes and quality: Understanding the quality of opioid use disorder care
could benefit from the development of a set of standard performance measures with
respect to quality of opioid use disorder treatment and specifically for medication-
assisted treatment, potentially by leveraging information from EHRs, as well as the more
commonly used services and pharmacy claims. Standardized or systematic reporting of
treatment process measures (e.g., frequency of urinalysis, drug screens, dosing) or
patient-reported outcomes (e.g., abstinence, craving, illicit drug use) would be valuable.
• Treatment and outcomes for criminal justice populations: Linking criminal justice
and treatment services data sources can clarify the treatments being used in the criminal
justice system and continuity of care for individuals who leave the criminal justice
system. For instance, under Chapter 55, Massachusetts has aimed to link person-level
data on substance abuse treatment received by prisoners with mortality data to understand
whether treatment during incarceration reduces likelihood of experience a fatal opioid-
related overdose (The Commonwealth of Massachusetts, Executive Office of Health and
Human Services, Department of Public Health, 2017).
Table 5.2 highlights common data source strengths and limitations noted during stakeholder
discussions regarding opioid use disorder treatment. Key takeaways regarding the advantages
and limitations of various data source types include:
• Many national data sources, including claims data, EHR data, and national surveys, offer
insights into treatment need, treatment utilization, and treatment supply. Each source uses
different measures to assess these outcomes.
• Information on buprenorphine prescriptions and buprenorphine-waivered prescribers is
available through several data sources, but using these data may entail costs.
30
Furthermore, these data provide information on only one type of treatment for opioid use
disorder.
• State-level information on treatment admissions for opioid use disorder and facilities
providing treatment for opioid use disorder are publicly available through national
treatment facility surveys. The quality of admissions data varies across states and over
time.
• Many of these data sets can be triangulated at the county- or state-level to better assess
the overall picture of how treatment need aligns with treatment capacity. However, none
supports person-level linkages across different potential sources of treatment for the
general population.
31

Table 5.2. Commonly Referenced Data Sources for Understanding Treatment Need and Access
Data Type and
Example Sources
Strengths Limitations
!"##$%&'()*&)('#+*
• ,-.,/
• 0%12$3
4(%5$6+&(3
• 78#9:"38*;$()6:
• 41)6'9(8$%*(3<*'3&)1<$+*&(+:*9(8#$36
• =%$+&%'96'"3*<(6(*&(3*&(961%$*6:$*9"91)(6'"3*6%$(6$<
>'6:*?19%$3"%9:'3$
• !(3*$@(#'3$*9%"2'<$%A9(6'$36*&$3+1+$+
• ,3B"%#(6'"3*"3*&"#"%?'<'6'$+*(3<*"6:$%*9%$+&%'96'"3+
C$DEDF*"9'"'<+G
• H'#'6$<*'3B"%#(6'"3*"3*<'(E3"+$+F*"6:$%*:$()6:&(%$*16')'I(6'"3
• J$K1'%$+*6%'(3E1)(6'3E*"6:$%*<(6(*+"1%&$+*6"*(++$++*"9'"'<*1+$
<'+"%<$%*(3<*6%$(6#$36*(&&$++
• ,++1$+*6%(&5'3E*'3<'2'<1()+*"2$%*6'#$
• L9'"'<*1+$*<'+"%<$%*6%$(6#$36*'+*"B6$3*9%'2(6$*&(+:*9(8*(3<
6:1+*3"6*(99%"9%'(6$)8*&(961%$<*'3*&)('#+*(3<*'+*3"6*&(961%$<*(6
())*'3*9:(%#(&8*&)('#+
• !"+6+*6"*"?6('3
4$<'&('<*&)('#+*
• M(6'"3()*"%*+6(6$
4$<'&('<*<(6(
+"1%&$+
• !(3*)'35*:"+9'6()*(3<*9:(%#(&8*&)('#+
• 7"#$*+'3E)$A+6(6$*(3()8+$+*:(2$*)'35$<*6"*<$(6:
&$%6'B'&(6$*<(6(
• !(3*$@(#'3$*"9'"'<*1+$*<'+"%<$%*<'(E3"+'+
• ,3B"%#(6'"3*"3*&"#"%?'<'6'$+*(3<*"6:$%*9%$+&%'96'"3+
C$DEDF*"9'"'<+G
• L3)8*9%"2'<$+*'3B"%#(6'"3*"3*"3$*9"91)(6'"3*C4$<'&('<G
• N(6(*+8+6$#+*3"6*+$6*19*6"*6%(&5*9$"9)$*)"3EA6$%#*E'2$3
'3+1%(3&$*&"2$%(E$*6%(3+'6'"3+
• !(33"6*+$$*'B*%$&$'2'3E*"6:$%*91?)'&)8*B13<$<*+1?+6(3&$*(?1+$
6%$(6#$36
• N'(E3"+'+*&"<$+*?'))$<*B"%*<"*3"6*3$&$++(%')8*%$B)$&6*(&61()
<'(E3"+'+
O;J*
• ;$()6:!"%$
,36$E%(6$<
J$+$(%&:
N(6(?(+$
• P%"19*;$()6:
!""9$%(6'2$
• !(3*&(961%$*3"3#$<'&(6'"3*6%$(6#$36*C$DEDF
9+8&:"+"&'()*6:$%(98G
• /?)$*6"*%$2'$>*9(6'$36*6$+6*%$+1)6+F*:'+6"%8F*<'(E3"+$+F
(3<*9)(3+*B"%*6%$(6#$36
• !)'3'&()*6$@6*:(+*%'&:*<(6(*"3*9%"E%$++*(3<*9%"?)$#+
6:(6*"B6$3*)(&5*,36$%3(6'"3()*!)(++'B'&(6'"3*"B*N'+$(+$+
&"<$+
• N$6(')$<*'3B"%#(6'"3*"3*9('3F*&"#"%?'<'6'$+F
+8#96"#+
• 41)6'9)$*)(>+*%$E(%<'3E*&"3B'<$36'()'68Q9%'2(&8*9%$&)1<$*(&&$++
6"*&)'3'&'(3*3"6$+
• N(6(*K1()'68*'+*6'$<*6"*<(6(*$36%8F*(3<*6:$%$*(%$*2(%'"1+*<(6(A
$36%8*'++1$+
• ,3*#(38*6%$(6#$36*+8+6$#+F*"3)8*:()B*"B*())*9%"2'<$%+*:(2$
(<"96$<*O;J+
32

Data Type and
Example Sources
Strengths Limitations
;"1+$:")<*+1%2$8+*
• M7NR;
• MO7/J!
• M(6'"3()*<(6(
• J'&:*'3B"%#(6'"3*"3*#$36()*:$()6:*(3<*+1?+6(3&$*1+$F
'3&)1<'3E*"9'"'<*#'+1+$*(3<*1+$*<'+"%<$%
• M7NR;*&"))$&6$<*(331())8
• M7NR;*"BB$%+*+"#$*'3+'E:6+*"3*<'2$%+'"3
• M7NR;*STUV*%$<$+'E3*:(+*'3B"%#(6'"3*"3*(38
9%$+&%'96'"3*9('3*%$)'$2$%*1+$*C3"6*"3)8*#'+1+$G
• ;'+6"%'&())8*:(2$*3"6*'3&)1<$<*#$<'&(6'"3+*1+$<*B"%*6%$(6#$36
• 7&%$$3*B"%*1+$*<'+"%<$%*+8#96"#+F*?16*<"*3"6*(+5*(?"16
B"%#()*<'(E3"+'+
• M"*#$(+1%$*"B*&(%$*K1()'68F*6%$(6#$36*%$6$36'"3
• 7(#9)$*#(8*#'++*:'E:A%'+5*E%"19+*C$DEDF*:"#$)$++F*(%%$+6$$+G
• MO7/J!*3"6*&"))$&6$<*(331())8*"%*#(<$*%$(<')8*(2(')(?)$*6"
%$+$(%&:$%+
• 76(6$*'<$36'B'$%+*%$+6%'&6$<
0%$(6#$36*B(&')'68*
+1%2$8+*
• 0ON7A/
• MA77/07
• M(6'"3()*<(6(*"3*(<#'6+*6"*6%$(6#$36*(3<*91?)'&
+$&6"%*+9$&'()68*&(%$
• R9*6"*6:%$$*<%1E+*"B*(?1+$*)'+6$<
• ,3B"%#(6'"3*"3*%$B$%%()*+"1%&$*C$DEDF*&%'#'3()*W1+6'&$
+8+6$#G
• MA77/07*'3&)1<$+*91?)'&*(3<*9%'2(6$*B(&')'6'$+*(3<
#$(+1%$+*"B*&(9(&'68
•
4$<'&(6'"3(++'+6$<*6%$(6#$36*'+*%$9"%6$<*(+*(*+'3E)$*2(%'(?)$
'3*0ON7*+"*&(33"6*<'BB$%$36'(6$*?$6>$$3*?19%$3"%9:'3$*(3<
#$6:(<"3$*0ON7*"3)8*'3&)1<$+*(E"3'+6*6%$(6#$36+
• H'#'6$<*'3B"%#(6'"3*"3*9(8#$36
• -1()'68*&"36%")*'++1$+*>'6:*0ON7F*(+*+6(6$+*#(8*3"6
&"3+'+6$36)8*(++$++*<(6(*K1()'68*"%*%$9"%6*"3*+'#')(%*9(6'$36+
• 0ON7*<"*3"6*'3&)1<$*9%'2(6$*B"%A9%"B'6*B(&')'6'$+
=%"2'<$%*&$3+1+*
• 7/4;7/
<(6(?(+$
• NO/*/!7/
• 4$(+1%$+*+199)8Q&(9(&'68*"B*>('2$%$<*9:8+'&'(3+
• !(3*)'35*6"*6:$*/#$%'&(3*4$<'&()*/++"&'(6'"3
=:8+'&'(3*4(+6$%B')$
• P$"E%(9:'&*<$6(')
• !"+6+*6"*"?6('3*NO/*/!7/
• 7/4;7/X+*91?)'&)8*(2(')(?)$*<(6(*+$6*&(961%$+*(%"13<*VV
9$%&$36*"B*9:8+'&'(3+
33
Better Targeting of Overdose1Reversing Drugs
Common themes emerging from discussions related to promoting use of overdose-reversing
drugs include the following:
• Naloxone distribution: Data about naloxone distributed outside of standard outpatient
pharmacy channels would help to identify capacity problems and ways to get naloxone to
the right individuals. There have been several case studies of OEND programs (Doyon et
al., 2016), but data on naloxone distribution through such programs are not systematically
collected or made publicly available.
• Naloxone effectiveness: Better data on the circumstances surrounding overdoses and
naloxone reversals would improve our understanding of under what circumstances and
how frequently naloxone fails to reverse an overdose. These data could also inform
efforts to modify naloxone use in communities facing increased fentanyl or carfentanil
overdoses. EMS data may be of particular value in this area.
• Treatment for individuals receiving naloxone: Linking individual-level naloxone
administration data with health care utilization data would improve our understanding of
the emergency department services and subsequent opioid use disorder treatment
provided to individuals receiving naloxone.
Table 5.3 highlights common data source strengths and limitations of data sources noted
during stakeholder discussions regarding naloxone access and use. Key takeaways regarding the
advantages and limitations of various data source types include the following:
• Commercial claims data may help in understanding trends and geographic variation in
naloxone distribution through retail pharmacy channels; however, pharmacies are just
one of the sources through which naloxone is distributed.
• A national data source containing information on community-based OEND programs is
managed by the Harm Reduction Council, but these data are not publicly available.
• While EMS data through NEMSIS can offer valuable insights regarding EMS
administration of naloxone, these data cannot include state identifiers, serving as a barrier
to analyses of the effects of state policy on EMS use of naloxone.
• There are some sources of systematically collected data on state naloxone policies, which
enhances assessment of how such policies affect outcomes such as mortality; however,
few data sources capture policy implementation or variation in local regulations or
protocols.
• Opioid-related mortality is an important outcome to evaluate in this area but greater use
of EMS or hospitalization data—particularly if the sources could be linked—would offer
value in understanding the trajectories of individuals treated with naloxone.
34
Table 5.3. Commonly Referenced Data Sources for Understanding Naloxone Access
Data Type and Example
Sources
Strengths Limitations
Commercial claims
• IQVIA
• Measures pharmacy distribution of naloxone
• Information on prescriber specialty
• Data on formulation
• Only captures the distribution of naloxone via pharmacy
channel
• Does not capture purchase and distribution via state or
community programs
• Costs to obtain
Mortality data
• CDC WONDER
• NVSS MCOD
• National data on opioid overdose mortality
• Information on opioid type
• CDC WONDER is readily downloadable
• Lags in data availability
• Variation in quality of reporting detail on drug
involvement
OEND program data
• MA Opioid Overdose
Prevention Pilot Program
• Harm Reduction
Coalition
• Fills in some data gaps regarding naloxone
distributed via state or community programs
• Information on where sites located, number of kits
distributed, etc.
• Not standardized
• National data not systematically collected or updated
• Not publicly available
EMS data
• NEMSIS
• Naloxone administration is reportedly a fairly high-
quality variable, and NEMSIS offers a Public
Naloxone Administration Dashboard (NEMSIS,
undated)
• Standardized collection of 911 call, incident, and
transport information across multiple EMS agencies
• Can do small-area analysis
• Not a registry of patients receiving care
• Data quality differs across agencies/states
• Some measures restricted
• No diagnosis information
• Barriers to linking or accessing geographic identifiers
Policy data
• PDAPS
• NPHL
• Legal databases
• Information on state policies to increase naloxone
access or use
• Can be merged at the state level with other data on
opioid-related outcomes
• Variation in naloxone-related regulations between
states may not be fully captured
• Data on EMS protocols not readily available
• Some historical data may not be provided in readily
analyzable formats
35
Better Data
Common themes emerging from discussions related to strengthening data to improve public
health surveillance include the following:
• Understanding the dynamic opioid ecosystem: The opioid crisis is a dynamic system
with multiple agents and networks of interacting individuals and agencies (Wakeland et
al., 2015; Burke, 2016). Greater efforts are needed to model and understand the dynamics
of the crisis, network patterns (e.g., prescriber, patient) at play, as well as macro-level
factors (e.g., sociological, economic, technological) involved. Such analyses would
require leveraging multiple data sources, including data about users of illicit opioids and
the illicit drug market. For instance, drug-seizure and drug-testing data from the National
Forensic Laboratory Information System (NFLIS) or System to Retrieve Information
from Drug Evidence (STRIDE), both managed by the DEA, contain product-specific data
on substances secured in law enforcement operations (see Table 5.4).
• Early warning signs of problematic use or problematic prescribing: Linking PDMP
data with outcomes data (e.g., hospital discharge, emergency department visit, treatment,
death, or criminal justice data) can facilitate development and validation of risk indicators
for opioid analgesic misuse, diversion, and/or potential overdose. For instance, one study
validated prescriber risk indicators derived using PDMP data by linking prescriber-level
data from Maine’s PDMP with data on medical board actions to assess how well their
prescriber risk indicators predicted likelihood of receiving a disciplinary action (Kreiner
et al., 2017).
• Detail on drugs involved in overdoses: Improved standardization across local
jurisdictions regarding testing for and recording specific drugs and drug types during
autopsies would enhance the consistency, validity, and reliability of information about
drug-related overdose deaths (Ruhm, 2017). Data about nonfatal overdose may also help
fill gaps in knowledge, although data costs are a potential barrier.
• Near-real-time data collection and access: Timely collection and access to data are
necessary to keep pace with the rapid evolution of the crisis, would facilitate
understanding emerging developments and local variation in the illicit supply of opioids,
and may facilitate timely responses. Other opportunities for surveillance could include
ways to leverage novel data sources (e.g., analysis of social media, the Dark Web,
wastewater analysis) to produce near-real-time insights (Kalyanam and Mackey, 2017;
Kalyanam et al., 2017).
Table 5.4 highlights common data source strengths and limitations noted during stakeholder
discussions regarding public health surveillance. Key takeaways regarding the advantages and
limitations of various data source types include the following:
• Each data source has notable strengths in identifying product-specific abuse or risk,
understanding interactions between licit and illicit markets for opioids and providing
timely information for surveillance and monitoring.
• Stakeholders noted common challenges that may limit the use of such data sources by
researchers. These include barriers to access (e.g., high costs, no explicit documentation
36
on how to access) and barriers to analyses (e.g., data files not provided in computable
formats, absence of unique identifiers).
• Some data sources, such as ADAM, that could offer insights on drug use and treatment
among high-risk populations are no longer fully operational.
37

Table 5.4. Commonly Referenced Data Sources for Understanding the Epidemic Through Better Public Health Surveillance
Data Type and
Example
Sources
Strengths Limitations
Mortality data
• NVSS MCOD
• Detail on drugs involved in overdose death
• Information on cause of death
• Complete census of deaths over time
• Access to microdata is limited
• Can be difficult and cumbersome to download
• Variation in quality of reporting detail on drug involvement
• Reporting delays
Prescription drug
monitoring data
• PDMP
• PBSS
• ARCOS
• Comprehensive data on distribution (ARCOS) and
prescribing (PDMP)
• Not restricted to one payer
• PDMPs can be used to develop measures around patient,
prescriber, and pharmacist risky behaviors
• Access barriers
• ARCOS not available in computable formats (i.e., only in
PDF form)
• Many states have capacity issues that limit ability to link
PDMP data with other data sources
• Many PDMPs do not collect unique IDs or have errors in
ID entry, creating technical issues in matching at the
individual level
National surveys
• ADAM
• Captures a high-risk population (arrestees)
• Has urinalysis results in addition to self-reported drug use
• Collects drug market information (e.g., drug acquisition
and payment)
• Collects information on substance abuse treatment history
• No longer fully operational
• Limited to few sites collecting data
• Recent data limited to adult male arrestees
Drug arrest data
• Criminal justice
agencies
• Could be used to examine network patterns of co-arrests
• If linked with other data, could be used to examine
systematic histories leading to arrest or indications of
diversion-related behaviors
• Often not available in electronic form that is usable
• Often difficulties in obtaining permissions to use data
Nonfatal
overdose data
• NPDS
• RADARS
• Captures broader set of overdose incidents than fatalities
• Detailed product- and drug-specific information
• Near-real time data
• Can analyze at local level
• RADARS has additional programs capturing measures of
diversion, use, street price
• Must be requested and purchased
• Data availability lags may vary by poison center
• High costs to obtain
38
Data Type and
Example
Sources
Strengths Limitations
Drug seizure and
drug testing data
• National
Forensic
Laboratory
Information
System (NFLIS)
• STRIDE
• Data on illicit drug supply, prices (STRIDE), and purity
• Product-specific information
• Seizure data generally available with less lag time
• Useful for assessing prevalence and location of emerging
drugs
• Access barriers
• Summary data may be available but are not generally
provided at the substate level
• Some drugs seized by law enforcement are not analyzed
by participating laboratories
39

6. Challenges and Opportunities for Implementing Successful
Data-Linking Strategies
Most of our findings from the environmental scan, data inventory, and stakeholder
discussions were applicable across the five-part HHS strategy. Thus, we do not structure our
discussion in this section around Strategic Priorities; rather, we identify general opportunities to
improve data quality and data linkages to enhance the ability of researchers to answer questions
related to the opioid crisis.
In the next section, we present nine key observations about challenges to data linkage or
analyses that emerged from our study. After each, we describe approaches that could potentially
help to reduce the challenge(s).
Key Observation 1
To advance research studying the effects of changes in state policies related to opioids, the
absence of national data collected in a standardized manner across states can limit the rigor and
robustness of potential analyses. While there are various state-based data initiatives aimed at
synthesizing data from different agencies into one data warehouse, national standards that align
states’ reporting in existing data systems would allow for nationally representative policy
studies. Barriers to research could also be lowered by ensuring that collected data are recorded
and made available in usable formats that support empirical analyses. There is thus a benefit to
be gained from standardizing how data currently being collected are recorded, reported, and
made available.
• Approach 1.1: Establish national standards on data collection and reporting for
currently available data sources. Challenges identified by stakeholders included limited
information on individuals who overdose and are attended to by EMS personnel but
decline transport to the hospital so are generally not captured in administrative claims
data. Thus, one approach may be to further encourage high-quality reporting by EMS
providers of a standardized set of information (Becknell and Simon, 2016) that would
ultimately flow up to state health systems and systems such as NEMSIS. Another
challenge identified was variation in reporting quality to TEDS across states and, over
time, such as variation in what states determine are eligible reporting facilities, what
counts as a treatment episode, and what data elements are required for reporting. This
variation may indicate a need to promote standardized high-quality reporting by states
and to establish improved documentation of potential differences across states and over
time in reporting to TEDS. Data transparency can be further enhanced by supporting the
development and dissemination of a data inventory for opioid research, accompanied by
appropriate technical documentation outlining the contents, characteristics, quality, and
potential limitations of individual data sets. This could be modeled similarly to the new
U.S. Census Bureau Data Repository (U.S. Census Bureau, 2017).
40
• Approach 1.2: Enhance data usability by ensuring that available data are provided
in readily analyzable formats. A substantial barrier noted by stakeholders was that
useful data sources are sometimes provided in formats (e.g., PDF formats) that do not
readily support empirical analyses. Examples include the ARCOS data, as well as state
data made available in a PDF table even though it had originally been created in Excel.
Having to work with such formats creates a cost for researchers, who must translate data
into a format that can be analyzed with statistical programs. The translation creates
unneeded risk of further data-entry errors. For data that are already being made available,
and particularly for data that may already exist in formats that facilitate incorporation into
analytic software, costs to researchers can be reduced by ensuring that data are provided
in files that support analysis is a straightforward way.
• Approach 1.3: Establish standardized performance measures for quality of
treatment processes and outcomes and encourage state treatment programs to
report on these measures. There are several challenges in developing performance
measures, including the need for rigorous assessment of their importance, feasibility, and
validity. One potential opportunity comes from EHRs which, depending on the quality of
the information contained within, may provide an opportunity to collect more in-depth
information, facilitate text mining of clinicians’ notes (e.g., through natural language
programming), and provide ongoing data collection during the course of treatment
(Garnick et al., 2012). Developing such standardized measures or guidelines for quality
of opioid use disorder treatment would facilitate assessment of which efforts effectively
improve access to treatment and recovery services while maintaining high-quality care.
Systematically reporting on the measures would enhance provider accountability and
provide evidence on treatment quality. Some states have already taken steps in this area.
For instance, Vermont has created a public dashboard that includes comparative
reporting on each of its treatment service “spokes,” and Rhode Island requires that
medical homes within opioid treatment programs track performance data (Boss, 2017)
and report data to the state to receive an enhanced payment rate (Chalk and Mark,
2017). Process-related measures of care and patient-centered outcomes data would be
valuable for understanding not just treatment utilization but quality of care.
Key Observation 2
Stakeholders consistently noted the particular value of state all-payer claims databases and
criminal justice data.
• Approach 2.1: Enhance researcher use of all-payer claims databases. While all-payer
claims data are not available for all states, stakeholders highlighted their benefits in
potentially capturing health care claims across an entire state’s population, allowing
studies to track utilization and compare rates across different populations with different
types of insurance. By making these data accessible and comparable in a single source,
all-payers claims data may be less costly to obtain or burdensome to analyze, compared
with obtaining and analyzing data from many different claims data sources. Furthermore,
some states (e.g., Massachusetts) have expended significant resources to enable record
linkages across payers (The Commonwealth of Massachusetts, Executive Office of
Health and Human Services, Department of Public Health, 2017), which potentially
offers a key advantage over other claims data sources (Dworsky, 2017). It is also worth
41
noting that Massachusetts’ all-payer claims database forms the spine of their Chapter 55
data system (discussed further in Approach 9.1) to enable linkages across multiple
interagency data sources (The Commonwealth of Massachusetts, Executive Office of
Health and Human Services, Department of Public Health, 2017).
Research could potentially be enhanced by promoting awareness of the benefits of such data
sources, socializing best practices for their creation and use in research, and making resources
available to increase awareness and prompt greater use by the research community. Many
discussants believed that significant benefits could be gained by encouraging more states to
create such databases and to make them more available to researchers while maintaining fidelity
to confidentiality and privacy requirements. However, self-insured plans can opt out of APCDs,
a significant limitation in examining the employer insured market (U.S. Supreme Court, 2016).
• Approach 2.2: Encourage incorporation of criminal justice data into public health
research. Person-level linkages of public health data sources (e.g., death records,
PDMPs, treatment facility data) with criminal justice data on arrests, incarcerations, or
treatment within the criminal justice system could be of value. Prior research has
obtained de-identified data that link state administrative data on clients receiving publicly
funded substance abuse treatment in specialty settings to arrest and incarceration data
from state criminal justice agencies (Acevedo et al., 2015; Garnick et al., 2014). Small-
area analyses of drug-seizure data complemented by analyses of detailed drug-overdose
data could also inform our understanding of illicit drug markets and supply-side
dynamics and may be less challenging to implement than person-level linkages.
One way to implement this approach would be a research partnership to develop data
systems focused on the criminal justice system and the opioid crisis and to potentially
provide researchers with de-identified files that would support analyses at the level of
fine geographic detail. At the state level, a recently published study (DeHart and Shapiro,
2016) offers insights into the implementation and use of integrated criminal justice and
public health data in South Carolina (DeHart, 2015). Further efforts in this area could
advance our understanding of treatments being used in the criminal justice system and
continuity of care for individuals who leave the criminal justice system; factors that
precede or follow criminal justice involvement related to opioids; and the evolution or
dynamics of illicit opioid markets and illicit opioid use.
Key Observation 3
Stakeholders noted that some data that were useful in strengthening public health
surveillance or capturing high-risk populations are no longer being collected (e.g., Drug Abuse
Warning Network [DAWN], ADAM). In addition, there are current data-collection efforts that
are well-positioned to collect measures relevant to the opioid crisis but historically have not
captured that information or are currently not making the information readily available to
researchers.
• Approach 3.1: Support reinstitution of useful data sets no longer being collected.
Stakeholders noted that the arrestee interview and drug-testing data collected through
42
ADAM provided insights not offered through other household surveys. Furthermore,
ADAM provided a national data source on individual users’ consumption and
expenditures, which offered valuable information on illicit drug markets. DAWN
provided a vital source of information on emergency room visits at the local level.
Bringing back and improving these data sets could help fill gaps in our understanding of
the opioid crisis. SAMHSA is planning to release an improved replacement of DAWN
(i.e., SAMHSA’s Emergency Department Surveillance System) (SAMHSA, 2016), and
research could be enhanced by promoting awareness of its value and supporting its
analysis.
• Approach 3.2: Augment existing federal data collections to capture information
relevant to the opioid crisis and facilitate researcher access to such data. Federally
funded surveys that are collected annually could incorporate new data elements or new
modules relevant to the opioid crisis. For instance, the NSDUH could begin collecting
information on pain, pain treatment, or diagnosis of opioid use disorder. TEDS could be
modified to include whether pharmacotherapy (and what types) is planned or offered at
discharge (Thomas et al., 2011). Interested researchers or other individuals could be
invited to propose new elements or modules to be incorporated into existing systems. To
maximize the benefits of these secondary data sources, there is a concurrent need to
facilitate researcher access to important but sensitive data elements (e.g., state identifiers
in the NSDUH are collected but are not widely available to researchers, and even
researchers with permission to use restricted NSDUH state identifiers have experienced
lengthy disruptions in access over the last several years).
Key Observation 4
An accessible source of consistent national data on opioid policies and strategies being
implemented is essential for evaluating the impacts of policies and initiatives. The Alcohol
Policy Information System (undated) is an exemplar of a rich source of policy data, providing
detailed state-by-state information for a variety of alcohol policies (and more recently for
cannabis policies). PDAPS (undated) offers an excellent source of policy data for state laws
related to PDMPs, naloxone access laws, Good Samaritan laws, and pain management clinic
laws. Continuing to expand and support such efforts in light of the rapidly evolving policy
environment offers a vital benefit to researchers evaluating the impact of state opioid policies
and initiatives.
• Approach 4.1: Support the construction and dissemination of a national database of
state policy and initiatives. Efforts in this area could include expanding the scope of
policies currently collected by systems such as PDAPS or developing new systems that
provide consistent information on state policies or efforts that have received less
evaluation (e.g., opioid prescribing policies, clinician education efforts, insurance policies
regarding reimbursement for pain treatment). Making data available on the timing of
policy enactment or implementation would also help support evaluations of the impact of
these interventions.
43
Key Observation 5
In the areas of treatment for opioid use disorder and use of naloxone, data currently being
used to understand and address these issues offer an incomplete picture. With respect to
treatment, there is not a strong set of measures that captures the extent to which treatment for
opioid use disorder is occurring through state block grants. With respect to naloxone, there is not
a strong set of measures available to track the distribution of naloxone through nonretail
pharmacies. This limits evaluation of the impact of naloxone programs and policies.
• Approaches 5.1: Support the systematic collection and availability of data on
individuals being treated through state block grants. Several data sources capture
information on prescriptions for buprenorphine and availability of treatment with
buprenorphine. However, stakeholders noted that administrative claims data fail to
capture individuals receiving treatment outside of the payment system (e.g., through state
block grants and community treatment programs). Additionally, better information is
needed about the population receiving treatment outside of the public sector (e.g., full
private facilities, which may still be subject to public credentialing). One approach to
begin filling this gap could be exploring ways to incentivize substance abuse treatment
programs to report to Medicaid using information about services provided for individuals
receiving capitated services (e.g., shadow claims) as occurs currently in some
jurisdictions. Careful consideration of patient privacy and confidentiality would be
critical in these efforts.
• Approach 5.2: Support reporting of and access to data on naloxone distribution
through nonpharmacy channels. While commercial claims data (e.g., IQVIA) can offer
insights on access to naloxone through outpatient retail channels, other important
distribution channels for naloxone are not captured in these data. These include naloxone
being provided to first responders directly through hospitals, grants, or other sources, as
well as naloxone being provided directly to the public through OEND programs and other
entities. Identifying methods to track naloxone being distributed through such channels,
such as working with manufacturers, is critical to developing a more comprehensive
understanding of the effectiveness of policies and initiatives seeking to enhance naloxone
distribution.
Key Observation 6
A fundamental need for linking data at the individual level is collection of individual
identifiers. Unique identifiers (e.g., social security numbers) have traditionally not been collected
or made available because of a number of regulatory and privacy concerns (Dokholyan et al.,
2009). Instead, linkages often rely on indirect identifiers (e.g., some combination of age, sex,
date of birth, geography). To accurately link data based on indirect identifiers, it is critical to
have matching algorithms that allow for the accurate extraction and utilization of meaningful
information, given the quantity and quality of the data elements available to link (Dusetzina,
Tyree, and Meyer, 2014).
44
• Approach 6.1: Support methodological research to develop improved algorithms for
matching individuals across and within data sources. Stakeholders noted a key barrier
for data linkages is that we cannot match as well as we need to across databases (or in
some cases, within databases). Identifying reasons for insufficient matching and
developing and validating improved matching algorithms is key to supporting data
linkages.
Key Observation 7
Mortality data are a key resource for both researchers and policymakers, but existing
collection and reporting efforts need to be improved. Up to 25 percent of all death certificates
fail to note the specific drug responsible for fatal overdose, and there are substantial geographic
disparities in rates of missingness (Ruhm, 2017). This reporting variation complicates both
research efforts and targeted enforcement or treatment efforts. Furthermore, stakeholders noted
that there are particular opportunities for linking mortality data with other sources, given the
more-limited confidentiality violations and hence lower privacy barriers in linking data once
someone is deceased (Code of Federal Regulations, 2009). The National Center for Health
Statistics has linked several surveys (e.g., the National Health Interview Survey) with death
certificate data from the NDI (CDC, 2018), although stakeholders noted that these data are
underused. Improving mortality data, leveraging these linking opportunities, and making linked
data more readily available could offer substantial progress toward better understanding opioid-
related harms.
• Approach 7.1: Support improved toxicology studies and reporting. The CDC has
expanded funding to help states and medical examiners improve data collection and
reporting for nonfatal surveillance and fatal overdose data and has funded states to
increase comprehensive toxicology testing (with 60 percent of this funding going toward
medical examiners and coroners). Facilitating access to these data will enhance the ability
of researchers and policymakers to better understand and respond to the rapidly evolving
opioid crisis by understanding trends such as the use of adulterants in illicit opioids.
• Approach 7.2: Support universal and timely reporting of overdose deaths by states and
encourage states to leverage interagency partnerships. Partnerships between departments
of public health, local police departments, emergency medical services, hospitals, and
other agencies could enhance the ability of states to obtain complete and timely
information on overdose deaths in the community. Combined with improved toxicology
studies and reporting, these efforts could support targeted interventions to aid community
organizations, law enforcement, public health agencies, and the broader general public.
• Approach 7.3: Enhance linkage mortality data to other data sources and promote their
use by researchers. Stakeholders noted that a key opportunity for advancing our
understanding of the opioid crisis is linking Medicaid claims with mortality data. One
approach could be to develop standards and requirements for data sharing by state
agencies overseeing Medicaid data and mortality data. Stakeholders mentioned prior
efforts to link CMS claims data with mortality data that were supported by the American
Recovery and Reinvestment Act of 2009, but it is unclear to what extent such initiatives
have been undertaken in more recent years. Given the scope of the opioid crisis, there
45
may be greater value in supporting such linkages than there has been historically. Such
linkages can enhance policy and program evaluations; for instance, the U.S. Census
Bureau Center for Administrative Records Research and Application, in partnership with
Chapin Hall at the University of Chicago and supported by the Laura and John Arnold
Foundation, are promoting research studies and methods for combining data across
agencies and levels of government to advance evidence-based policymaking (Goerge,
Gjertson, and De La Cruz, 2017).
Key Observation 8
Effective responses to the rapidly evolving opioid crisis rely on the timely collection,
reporting, and analyses of crucial health information. Near-real time data collection at fine
geographic detail can support identification of high-risk locations and help inform timely and
effective community interventions. Several states have made great strides toward improving the
speed at which data on nonfatal overdose are collected and analyzed.
However, there are several challenges with near-real time surveillance systems. Substantial
costs and resources are required to implement and manage such systems, and the costs of data
management and analysis increase as systems receive increasing amounts of data with increasing
speed and diversity. Stakeholders also mentioned that laws governing the process by which data
collection occurs, such as the Paperwork Reduction Act, may also create substantial lags in
starting up new data collection efforts for surveillance, as the time to obtain the requisite
permissions often exceeds six months. Near-real time data collection also suffers from greater
data-quality challenges compared with data collection that occurs over a longer time frame, and
potential issues with record completeness and accurate processing and transmission necessitate
ongoing monitoring and communication (Ising et al., 2016). Despite these challenges, there is
significant public health value to be gained by supporting the collection and analysis of such
systems.
• Approach 8.1: Use evidence on innovative state or local approaches to develop and
utilize near-real time surveillance systems to advance the use and operations of such
systems more broadly. Many existing state approaches to near-real-time surveillance
systems leverage data on nonfatal overdoses (Box 5). Evidence on how states have used
these systems, challenges faced in their implementation and use, and insights about how
challenges have been overcome can be used to support the development of near-real-time
surveillance tools in other jurisdictions.
• Approach 8.2: Support innovative research on the use of nontraditional data
sources (e.g., social media, the Dark Web) to inform public health action. There has
been increasing research interest in methods to mine and analyze nontraditional data
resources to bolster public health surveillance. Studies have analyzed Twitter messages
and web forum postings to understand various forms of opioid misuse and prescription
drug diversion (Katsuki, Mackey, and Cuomo, 2015; Anderson et al., 2017; Chan, Lopez,
and Sakar, 2015), used Google trends data to forecast state-level mortality (Parker et al.,
2017), and used information from cryptomarket forums on the Dark Web to assess
emerging trends in new psychoactive substances (Van Hout, Claire, and Hearne, 2017).
46

While cryptomarkets represent only a slice of the total illicit drug trade, studies have used
web crawlers to scrape cryptomarket listings, vendor profiles, and forum discussions to
map online illicit drug distribution networks, assess prevalence and trends in the illicit
online sales and prices of different drug types, and identify emerging drug trends (see
Barratt and Aldridge, 2016, for an overview of challenges and opportunities in
cryptomarket research) (Van Hout, Claire, and Hearne, 2017; Bhaskar, Linacre, and
Machin, forthcoming, Ladegaard, 2017; Broséus et al., 2016). Advancing methods to
harness these data sources as a public health surveillance tool can offer a key resource for
identifying risks and emerging trends (Brownstein et al., 2009).
Box 5. Examples of State Efforts to Develop and Use Near-Real Time Surveillance
systems
Rhode Island’s Opioid Overdose Reporting System is a flexible near-real time
surveillance system that compiles information on cases of opioid overdose from the state’s
hospitals and emergency departments, although noted challenges have included incomplete
compliance with reporting requirements (McCormick, Koziol, and Sanchez, 2017).
North Carolina’s statewide syndromic surveillance system (the North Carolina Disease
Event Tracking and Epidemiologic Collection Tool) provides near-real time collection and
analysis of statewide emergency department data, poison center call data, and emergency
medical services data (Ising et al., 2016).
Key Observation 9
PDMPs are a valuable resource for understanding the opioid crisis, and there is significant
value to be gained by linking PDMPs with a variety of other data sources. All states now operate
a PDMP system (PDMP, Training and Technical Assistance Center, 2017), but they vary
substantially in data collection, reporting, and interoperability (Pardo, 2017; Manasco et al.,
2016). Developing a complete and consistent PDMP data set for analysis is essential for studying
prescription drug abuse. Linking these data with public health and criminal justice data sources
would support public health surveillance of opioid-related problems. Several state-level efforts,
often organized around PDMPs, are underway toward developing individual-level data linkages
across multiple data sources, and there is an opportunity for further partnerships between federal
and state agencies to support such efforts and invest in making these state linkages more useful
to research and practice. An exemplar of such an approach is the Bureau of Justice Assistance’s
Harold Rogers PDMP, which supports local, state, or regional collaborative efforts to collect and
analyze multiple sources of data. The program’s goal is to enhance understanding of the opioid
crisis and develop data-driven strategies to support surveillance, treatment, and prevention
efforts for at-risk individuals (Paulozzi, Kilbourne, and Desai, 2011).
47

• Approach 9.1: Use evidence on innovative state approaches to leveraging and
linking PDMP systems to publish guidance and recommendations on how states can
support linking PDMP data with other data sources. Given regulatory and
confidentiality concerns, it may be helpful to explore if a directive could be issued
indicating that there should be no attempts to subpoena PDMP data (or associated data
linked to PDMPs) for federal investigations. Establishing guidance for allowing
researchers controlled access to de-identified linked data could further promote the value
of linking PDMP with other sources, particularly if de-identified statistical data from
multiple states could be made available through a single federal or federally supported
source, such as the Brandeis PBSS. Some examples of state PDMP data-linkage efforts
are highlighted in Box 6.
There is also substantial interest in linking PDMP data with social services data (e.g., child
welfare data) to better understand how opioid misuse affect child welfare outcomes. While our
stakeholder discussion did not identify states that are currently making these linkages, a recent
study linked county-level data on controlled substance prescriptions rates from Florida’s Drug-
Related Outcomes Surveillance and Tracking System with county-level data on child removal
rates (Quast, Storch and Yampolskaya, 2018), and developing broader data linkages to support
analyses of the effects of the opioid crisis on children and families is an area to consider
supporting.
Box 6. Examples of Approaches to Linking PDMP Data with Other State Sources
Washington state links PDMP data to the state’s Medicaid and Worker’s Compensation
claims data through Washington State’s Data Sharing Initiative with Medicaid and Workers’
Compensation (PMDP, 2013).
Massachusetts is a noted example of state success in linking PDMP data to a broad range
of other public health and criminal justice data sources. Chapter 55 of the Acts of 2015
permitted the linkage and analysis of several government data sources to inform
programmatic decisions, guide the development of policies, and advance understanding of
the opioid crisis. Under Chapter 55, Massachusetts’ Department of Public Health has
connected (in most cases, at the individual level) ten data sources managed by five state
agencies to develop a data warehouse structure. The system also collects community-level
data on naloxone (e.g., enrollments, refills, and rescues through the Massachusetts
Department of Public Health Naloxone program), drug seizures, and socioeconomic and
demographic characteristics (The Commonwealth of Massachusetts, Executive Office of
Health and Human Services, Department of Public Health, 2017).
Maryland is another example of a state that has overcome interpretational challenges of
42 CFR Part II (establishing special privacy protections for health care records related to the
treatment of substance use disorders) and is currently advancing efforts to link person-level
data from the PDMP, drug use and alcohol treatment admissions, hospital admissions,
fatalities investigated by the medical examiner, and criminal justice data (Saloner, 2016;
Lyons, 2017).
48
• Approach 9.2: Encourage states to improve PDMP systems to ensure data
compatibility with other states. Standardization of electronic data collection for key
elements for all state PDMPs would facilitate cross-state sharing and collaboration with
other agencies (e.g., Medicaid, Department of Veterans Affairs). The American Society
for Automation in Pharmacy guidelines created a PDMP standard for reporting, most
recently updated in 2016 (American Society for Automation in Pharmacy, 2016).
Encouraging states to use the most recent version of the guidelines could support
interoperability and comprehensive data analysis (Greenwood-Ericksen et al., 2016). To
enhance interstate accessibility of PDMPs, one approach could include legislation
enabling sharing between PDMPs in all states.
Summary
Significant work is being done at the federal, state, and local level to combat the opioid
crisis. There has also been a substantial increase in research that has improved our understanding
of the complex and multidimensional nature of the opioid crisis, and that has advanced the
evidence base regarding the effectiveness of opioid policies and initiatives to reduce opioid-
related harms. There are significant resources within reach for the use and analysis of secondary
data, but not all are being taken advantage of. This report outlines a range of strategies that can
improve and promote available data to better understand the crisis.
Meaningful progress can be made on many of our potential approaches; doing so would
likely provide significant value to opioid policy researchers and inform policy developments.
Potential approaches that can be taken in the short-term include the following:
• Enhance data usability by ensuring available data are provided in readily
analyzable formats (Approach 1.2, Section 6): Progress over the short term can be made
by ensuring that publicly available data that are already electronically compiled in
analyzable formats are made readily accessible in machine-readable formats (e.g., CSV,
XML, ASCII) and by providing adequate technical documentation about important
aspects of the data. Entities publishing data in graphical format could enhance data
transparency by including links to analyzable formats of the data underlying the graphs.
• Support universal and timely reporting of overdose deaths by states and improve
toxicology reporting (Approach 7.2, Section 6): Progress over the short term can be
made in this area by updating best practices for coroners and medical examiners to report
overdose fatalities by disseminating such best practices and by continuing to support
improved toxicology reporting. Studies have supported that centralized medical examiner
systems have more-complete recording of specific drugs involved in drug intoxication
deaths compared with states with a decentralized county coroner system (Warner et al.,
2013).
• Use evidence on innovative state approaches to leverage and link PDMP systems to
publish guidelines and recommendations for states to support linking PDMP data
with other data sources (Approach 9.1, Section 6): There are several innovative state
approaches currently underway to link PDMP systems with other data sources—in some
cases, at the individual level. Short-term progress can be made in this area by developing
49
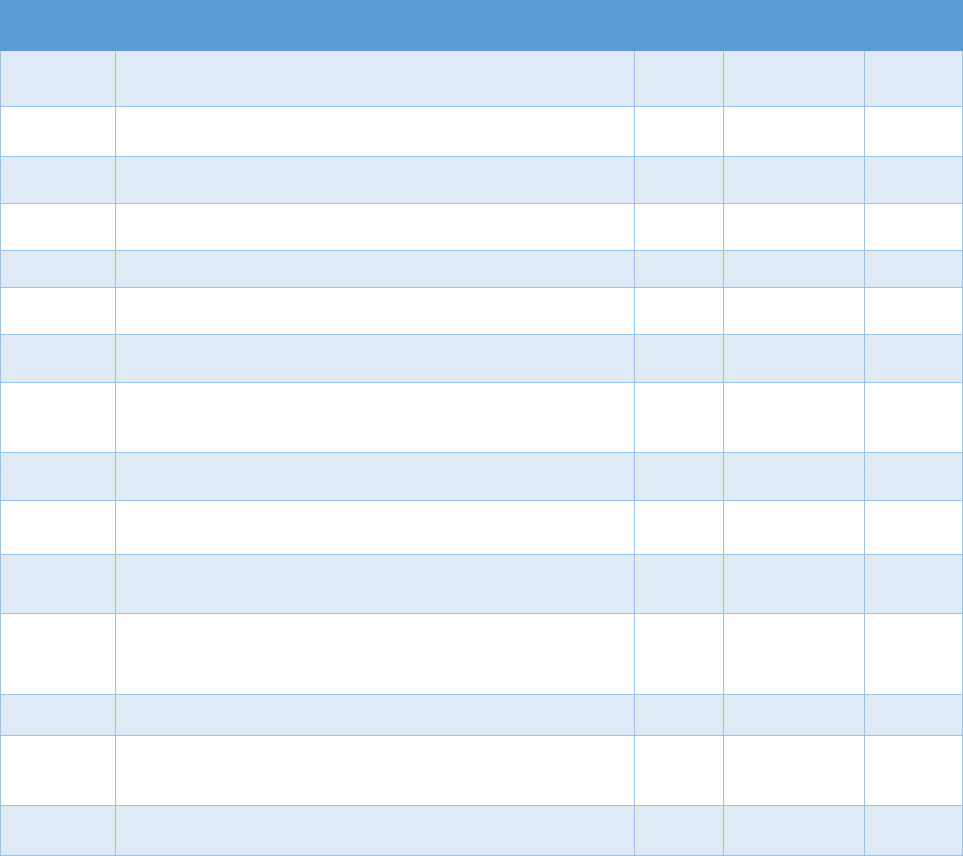
guidelines based on the challenges states have faced and how they have been overcome
and investing in making these linkages more useful to research and practice.
In Table 6.1, we provide an overview of timelines, based on conversations with stakeholders,
in which it may be possible to achieve meaningful progress toward the approaches discussed
above. We recognize, however, that there may be a range of complexities that stakeholders are
unaware of that may challenge meeting such aggressive timelines. Nevertheless, given the
human and societal toll of the opioid crisis and the potential benefits from additional high-quality
research that these approaches could support, we believe it is a public health imperative to create
and make available improved data assets that will support more informed efforts to address the
opioid crisis.
Table 6.1. Time Frame for Potential Approaches to Implementing Successful Data-Linking
Strategies
Approach Approach Description
Short
Term*
Intermediate
Term*
Long
Term*
1.1
Establish national standards on data collection and
reporting for currently available data sources.
X X
1.2
Enhance data usability by ensuring available data are
provided in readily analyzable formats.
X
1.3
Establish standardized performance measures for
quality of treatment.
X X
1.4
Encourage state treatment programs to report on
treatment processes and outcomes.
X X
2.1 Enhance researcher use of all-payer claims databases. X
2.2
Encourage incorporation of criminal justice data into
public health research.
X X
3.1
Support reinstitution of useful data sources no longer
being collected.
X X
3.2
Augment existing federal data collections to capture
information relevant to the opioid crisis and facilitate
researcher access to such data.
X X
4.1
Support the construction and dissemination of a
national database of state policy and initiatives.
X X
5.1
Support the systematic collection and availability of data
on individuals being treated through state block grants.
X X
5.2
Support reporting of and access to data on naloxone
distribution through nonpharmacy channels.
X X
6.1
Support methodological research to develop improved
algorithms for matching individuals across and within
data sources.
X X
7.1 Support improved toxicology studies and reporting. X X
7.2
Support universal and timely reporting of overdose
deaths by states and encourage states to leverage
interagency partnerships.
X X
7.3
Enhance linking mortality data to other data sources
and promote their use by researchers.
X X
50
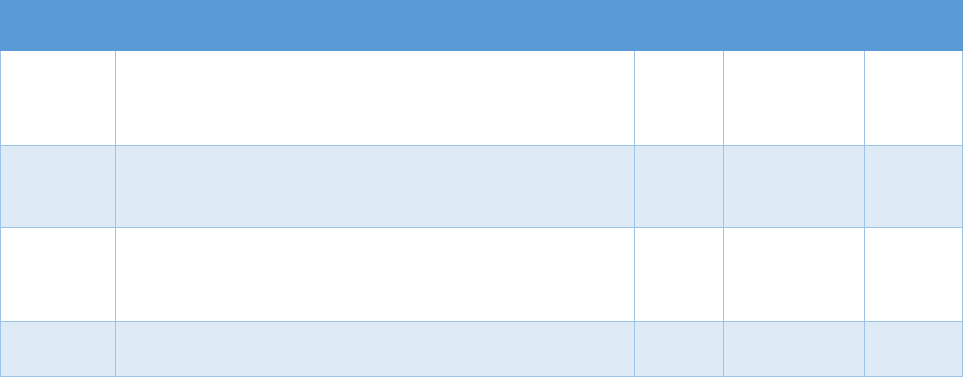
Approach Approach Description
Short
Term*
Intermediate
Term*
Long
Term*
8.1
Use evidence on innovative state or local approaches to
develop and utilize near-real time surveillance systems
to advance the use and operations of such systems
more broadly.
X X
8.2
Support innovative research on the use of nontraditional
data sources (e.g., social media, the Dark Web) to
inform public health action.
X
9.1
Use evidence on innovative state approaches to
leverage and link PDMP systems to publish guidelines
and recommendations on how states can support
linking PDMP data with other data sources.
X
9.2
Encourage states to improve PDMP systems to ensure
data compatibility with other states.
X X
* Short term = meaningful progress within six months; intermediate term = meaningful progress within 12 months;
long term = meaningful progress may take more than 12 months
51

References
Acevedo, Amdrea, Deborah W. Garnick, Robert Dunigan, Constance M. Horgan, Grant A.
Ritter, Margaret T. Lee, Lee Panas, Kevin Campbell, Karin Haberlin, Dawn Lambert-Wacey,
Tracy Leeper, Mark Reynolds, and David Wright, “Performance Measures and Racial/Ethnic
Disparities in the Treatment of Substance Use Disorders,” Journal of Studies on Alcohol and
Drugs, Vol. 76, No. 1, January 2015, pp. 57–67.
Agency for Healthcare Research and Quality, “Medical Expenditure Panel Survey,” webpage,
undated. As of July 6, 2018:
https://meps.ahrq.gov/mepsweb/
Agency for Healthcare Research and Quality, “Healthcare Cost and Utilization Project (HCUP),”
webpage, last updated June 2018. As of July 1, 2018:
https://hcupnet.ahrq.gov/#setup
Ahmsbrak, Rebecca, Jonaki Bose, Sarra L. Hedden, Rachel N. Lipari, and Eunice Park-Lee E,
Key Substance Use and Mental Health Indicators in the United States: Results from the 2016
National Survey on Drug Use and Health, Rockville, Md.: Center for Behavioral Health
Statistics and Quality, Substance Abuse and Mental Health Services Administration,
September 2017.
Albert, Su, Fred W. Brason II, Catherine K. Sanford, Nabarun Dasgupta, Jim Graham, and Beth
Lovette, “Project Lazarus: Community-Based Overdose Prevention in Rural North Carolina,”
Pain Medicine, Vol. 12, Supplement 2, June 2011, pp. S77–S85.
Alcohol Policy Information System, “Welcome to the Alcohol Policy Information System,”
webpage, undated. As of May 17, 2018:
https://alcoholpolicy.niaaa.nih.gov
American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders:
DSM-5, 5th ed. Arlington, Va.: American Psychiatric Publishing; 2013.
American Society for Automation in Pharmacy, “2016 ASAP Version 4.2A Standard for
Prescription Drug Monitoring Programs,” webpage, 2016. As of September 18, 2017:
https://www.asapnet.org/pmp-implementation-guides.html
amfAR, “Opioid and Health Indicators Database,” webpage, undated. As of May 17, 2018:
http://opioid.amfar.org/
Anderson, Daren R., Ianita Zlateva, Emil N. Coman, Khushbu Khatri, Terrence Tian, and Robert
D. Kerns, “Improving Pain Care Through Implementation of the Stepped Care Model at a
52

Multisite Community Health Center,” Journal of Pain Research, Vol. 201, No. 9, 2016, pp.
1021–1029.
Anderson, Laurie S., Heidi G. Bell, Michael Gilbert, Julie E. Davidson, Christina Winter,
Monica J. Barratt, Beta Win, Jeffery L. Painter, Christopher Menone, Jonathan Sayegh, and
Nabarun Dasgupta, “Using Social Listening Data to Monitor Misuse and Nonmedical Use of
Bupropion: A Content Analysis,” JMIR Public Health Surveillance, Vol. 3, No. 1, February
1, 2017, p. e6.
Asnsolabehere, Stephen, and Eitan D. Hersh, “ADGN: An Algorithm for Record Linkage Using
Address, Date of Birth, Gender, and Name,” Statistics and Public Policy, Vol. 4, No. 1,
2017, pp. 1–10.
Appari, Ajit, M. Eric Johnson, and Denise L. Anthony, “Meaningful Use of Electronic Health
Record Systems and Process Quality of Care: Evidence from a Panel Data Analysis of U.S.
Acute-Care Hospitals,” Health Services Research, Vol. 48, Part 1, April 2013, pp. 354–375.
Arizona Department of Health Services, “Opioid Epidemic: Real Time Opioid Data,” webpage,
date range June 15–2017–June 14, 2018. As of June 27, 2018:
https://www.azdhs.gov/prevention/womens-childrens-health/injury-prevention/opioid-
prevention/index.php
Baehren, David F., Catherine A. Marco, Danna E. Droz, Sameer Sinha, E. Megan Callan, and
Peter Akpuonu, “A Statewide Prescription Monitoring Program Affects Emergency
Department Prescribing Behaviors,” Annals of Emergency Medicine, Vol. 56, No. 1, July
2010, pp. 19–23.
Barratt, Monica J., and Judith Aldridge, “Everything You Always Wanted to Know About Drug
Cryptomarkets* (*but Were Afraid to Ask),” Internal Journal Drug Policy, Vol. 35,
September 2016, pp. 1–6.
Baumblatt, Jane A. Gwira, Caleb Wiedeman, John R. Dunn, William Schaffner, Leonard J.
Paulozzi, and Timothy F. Jones, “High-Risk Use by Patients Prescribed Opioids for Pain and
Its Role in Overdose Deaths,” JAMA Internal Medicine, Vol. 174, No. 5, 2014, pp. 796–801.
Becker, William C., Brenda T. Fenton, Cynthia A. Brandt, Erin L. Doyle, Joseph Francis, Joseph
L. Goulet, Brent A. Moore, Virginia Torrise, Robert D. Kerns, and Peter W. Kreiner,
“Multiple Sources of Prescription Payment and Risky Opioid Therapy Among Veterans,”
Medical Care, Vol. 55, July 2017, pp. S33–S36.
Becker, William C., David A. Fiellin, and Rani A. Desai, “Non-Medical Use, Abuse, and
Dependence on Sedatives and Tranquilizers Among U.S. Adults: Psychiatric and Socio-
Demographic Correlates,” Drug and Alcohol Dependence, Vol. 90, Nos. 2–3, October 8,
2007, pp. 280–287.
53

Becker, William C., Lynn E. Sullivan, Jeanette M. Tetrault, Rani A. Desai, and David A. Fiellin,
“Non-Medical Use, Abuse, and Dependence on Prescription Opioids Among U.S. Adults:
Psychiatric, Medical, and Substance Use Correlates,” Drug and Alcohol Dependence, Vol.
94, Nos. 1–3, April 1, 2008, pp. 38–47.
Becknell, John, and Lauren Simon, “Beyond EMS Data Collection: Envisioning an Information-
Driven Future for Emergency Medical Services,” Washington, D.C.: National Highway
Traffic Safety Administration, Report No. DOT HS 812 361, December 2016. As of April
13, 2018:
https://www.ems.gov/pdf/Beyond_EMS_Data_Collection.pdf
Bhaskar, V., Robin Linacre, Stephen Machin, “The Economic Functioning of Online Drugs
Markets,” Journal of Economic Behavior and Organization, forthcoming.
Blewett, Lynn A., and Michael Davern, “Meeting the Need for State-Level Estimates of Health
Insurance Coverage: Use of State and Federal Survey Data,” Health Services Research, Vol.
41, Part 1, June 2006, pp. 946–975.
Blondell, Richard D., Heather N. Dodds, Monica N. Blondell, and Danna C. Droz, “Is the
Kentucky Prescription Reporting System Useful in the Care of Hospitalized Patients?” The
Journal of the Kentucky Medical Association, Vol. 102, No. 1, p. 15-9.
Blumenthal, David, and Marilyn Tavenner, “The ‘Meaningful Use’ Regulation for Electronic
Health Records,” New England Journal of Medicine, Vol. 363, August 5, 2010, pp. 501–504.
Boscarino, Joseph A., Margaret Rukstalis, Stuart N. Hoffman, John J. Han, Porat M. Erlich,
Glenn S. Gerhard, and Walter F. Stewart, “Risk Factors for Drug Dependence Among Out-
Patients on Opioid Therapy in a Large U.S. Health-Care System,” Addiction, Vol. 105, No.
10, October 2010, pp. 1776–1782.
Boss, Rebecca, “Planning and Implementing Comprehensive MAT Service Deliver Models: A
Vision for Substance Use Disorder Treatment,” slide presentation, October 26, 2017. As of
April 13, 2018:
http://www.dhss.delaware.gov/dhss/dph/hsp/files/visconboss.pdf
Bradley, Cathy J., Bassam Dahman, and Charles W. Given “Inadequate Access to Surgeons:
Reason for Disparate Cancer Care?” Medical Care, Vol. 47, No. 7, July 2009, pp. 758–764.
Bradley, Cathy J., Lynne Penberthy, Kelly J. Devers, and Debra J. Holden, “Health Services
Research and Data Linkages: Issues, Methods, and Directions for the Future,” Health
Services Research, Vol. 45, Part 2, October 2010, pp. 1468–1488.
Brady, Joanne E., Hannah Wunsch, Charles DiMaggio, Barbara H. Lang, James Giglio, and
Guohua Li, “Prescription Drug Monitoring and Dispensing of Prescription Opioids,” Public
Health Reports, Vol. 129, No. 2, March–April 2014, pp. 139–147.
54

Broséus, J., Damien Rhumorbarbe, Caro Mireault, V. Ouellette, Frank Crispino, and David
Décart-Hétu, “Studying Illicit Drug Trafficking on Darknet Markets: Structure and
Organisation from a Canadian Perspective,” Forensic Science International, Vol. 264, July
2016, pp. 7–14.
Brownstein, John S. Clark C. Freifeld, and Lawrence C. Madoff, “Digital Disease Detection—
Harnessing the Web for Public Health Surveillance,” New England Journal of Medicine, Vol.
360, No. 21, May 21, 2009, pp. 2153–2155, 2157.
Broz, Dita, and Lawrence J. Ouellet, “Racial and Ethnic Changes in Heroin Injection in the
United States: Implications for the HIV/AIDS Epidemic,” Drug and Alcohol Dependence,
Vol. 94, Nos. 1–3, April 1, 2008, pp. 221–233.
Burke, Donald S., “Forecasting the Opioid Epidemic,” Science, Vol. 354, No. 6312, November
4, 2016, p. 529.
Burns, Rachel M., Rosalie L. Pacula, Sebastian Bauhoff, Adam J. Gordon, Hollie Hendrikson,
Douglas L. Leslie, and Bradley D. Stein, “Policies Related to Opioid Agonist Therapy for
Opioid Use Disorders: The Evolution of State Policies from 2004 to 2013,” Substance Abuse,
Vol. 37, No. 1, January–March 2016, pp. 63–69.
Campanella, Paolo, Emanuela Lovato, Claudio Marone, Lucia Fallacara, Agostino Mancuso,
Walter Ricciardi, and Maria Lucia Specchia, “The Impact of Electronic Health Records on
Healthcare Quality: A Systematic Review and Meta-Analysis,” European Journal of Public
Health, Vol. 26, No. 1, February 2016, pp. 60–64.
Campbell, Kevin M., Dennis Deck, and Antoinette Krupski, “Record Linkage Software in the
Public Domain: A Comparison of Link Plus, The Link King, and a ‘Basic’ Deterministic
Algorithm,” Health Informatics Journal, Vol. 14, No. 1, March 2008, pp. 5–15.
Capricorn, “Chicago Area Patient-Centered Outcomes Research Network,” website, undated. As
of May 17, 2018:
http://capricorncdrn.org
Carrell, David, Jack Mardekian, David Cronkite, Arvind Ramaprasan, Kristina Hansen, David E.
Gross, Roy E. Palmer, Elizabeth Masters, and Michael Von Korff, “A Fully Automated
Algorithm for Identifying Patients with Problem Prescription Opioid Use Using Electronic
Health Record Data,” Drug and Alcohol Dependence, Vol. 171, February 1, 2017, p. e36.
Centers for Disease Control and Prevention, “HIV Infection and HIV-Associated Behaviors
Among Injecting Drug Users—20 Cities, United States, 2009,” Morbidity and Mortality
Weekly Report (MMWR), Vol, 61, No. 8, March 2, 2012, pp. 133–138.
Centers for Disease Control and Prevention, “National Hospital Care Survey,” webpage, last
updated September 10, 2015. As of May 17, 2018:
https://www.cdc.gov/nchs/nhcs/about_nhcs.htm
55

Centers for Disease Control and Prevention, “Opioid Overdose: Opioid Data Analysis,”
webpage, last updated February 9, 2017. As of May 17, 2018:
https://www.cdc.gov/drugoverdose/data/analysis.html
Centers for Disease Control and Prevention, “Data-Driven Prevention Initiative (DDPI),”
webpage, last updated October 3, 2017. As of May 16, 2018:
https://www.cdc.gov/drugoverdose/foa/ddpi.html
Centers for Disease Control and Prevention, “National Health Interview Survey,” PDF, updated
November 9, 2017. As of May 17, 2018:
https://www.cdc.gov/nchs/data/datalinkage/LinkageTable.pdf
Centers for Disease Control and Prevention “Drug Overdose Death Data,” December 19, 2017.
As of April 9, 2018:
https://www.cdc.gov/drugoverdose/data/statedeaths.html
Centers for Disease Control and Prevention, “National Center for Health Statistics: Vital
Statistics Rapid Release, Provisional Drug Overdose Death Counts,” webpage, last updated
June 13, 2018. As of July 1, 2018:
https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
Centers for Disease Control and Prevention, “CDC WONDER,” webpage, updated June 27,
2018. As of July 1, 2018:
https://wonder.cdc.gov
Centers for Disease Control and Prevention, “NCHS Data Linked to NDI Mortality Files,”
webpage, June 28, 2018. As of July 1, 2018:
https://www.cdc.gov/nchs/data-linkage/mortality.htm
Centers for Medicare and Medicaid Services, “Chronic Conditions Data Warehouse (CCW),”
webpage, 2018. As of May 30, 2018:
https://www.ccwdata.org/web/guest/condition-categories#Proposed_OUD_Indicator
Cerdá, Magdalena, Andrew Gaidus, Katherine M. Keyes, William Ponicki, Silvia Martins,
Sandro Galea, and Paul Gruenewald, “Prescription Opioid Poisoning Across Urban and
Rural Areas: Identifying Vulnerable Groups and Geographic Areas,” Addiction, Vol. 112,
No. 1, January 2017, pp. 103–112.
Chalk, Mady, and Tami L. Mark, “Deploying the Cascade of Care Framework to Address the
Opioid Epidemic Means Taking a Closer Look at Quality Measures,” Health Affairs Blog,
June 21, 2017. As of September 18, 2017:
http://healthaffairs.org/blog/2017/06/21/deploying-the-cascade-of-care-framework-to-
address-the-opioid-epidemic-means-taking-a-closer-look-at-quality-measures
56

Chan, Brian, Andrea Lopez, and Urmimala Sarkar, “The Canary in the Coal Mine Tweets: Social
Media Reveals Public Perceptions of Non-Medical Use of Opioids,” PLoS One, Vol. 10, No.
8, August 7, 2015, p. e0135072.
Chou, Roger, P. Todd Korthuis, Melissa Weimer, Christina Bougatsos, Ian Blazina, Bernadette
Zakher, Sarah Grusing, Beth Devine, and Dennis McCarty, Medication-Assisted Treatment
Models of Care for Opioid Use Disorder in Primary Care Settings, Rockville, Md.: Agency
for Healthcare Research and Quality, AHRQ Publication No. 16(17)-EHC039-EF, December
2016.
Clark, D. E., “Practical Introduction to Record Linkage for Injury Research,” Injury Prevention,
Vol. 10, No. 3, June 2004, pp. 186–191.
Clark, Robin E., Mihail Samnaliev, Jeffrey D. Baxter, and Gary Y. Leung, “The Evidence
Doesn’t Justify Steps by State Medicaid Programs to Restrict Opioid Addiction Treatment
with Buprenorphine,” Health Affairs, Vol. 30, No. 8, August 2011, pp. 1425–1433.
Code of Federal Regulations, Title 45, Public Welfare, Department of Health and Human
Services, Part 46, Protection of Human Subjects, revised January 15, 2009, effective July 14,
2009.
Commission on Evidence-Based Policymaking, “The Promise of Evidence-Based Policymaking:
Report of the Commission on Evidence-Based Policymaking,” Washington, D.C., September
2017. As of June 27, 2018:
https://www.cep.gov/content/dam/cep/report/cep-final-report.pdf
The Commonwealth of Massachusetts, Executive Office of Health and Human Services,
Department of Public Health, An Assessment of Opioid-Related Deaths in Massachusetts
(2013–2014), September 2016.
The Commonwealth of Massachusetts, Executive Office of Health and Human Services,
Department of Public Health, “An Assessment of Fatal and Nonfatal Opioid Overdoses in
Massachusetts (2011–2015),” August 16, 2017. As June 27, 2018:
http://www.mass.gov/eohhs/docs/dph/stop-addiction/legislative-report-chapter-55-aug-
2017.pdf
Coorevits, Pascal, Mats Sundgren, Gunnar O. Klein, Anne Bahr, Brecht Claerhout, Christel
Daniel, Martin Dugas, Danielle Dupont, Andreas Schmidt, Peter Singleton, Georges De
Moor, and Dipak Kalra, “Electronic Health Records: New Opportunities for Clinical
Research,” Journal of Internal Medicine, Vol. 274, No. 6, December 2013, pp. 547–560.
Cunningham, Peter J., and Len M. Nichols, “The Effects of Medicaid Reimbursement on the
Access to Care of Medicaid Enrollees: A Community Perspective,” Medical Care Research
and Review, Vol. 62, No. 6, December 2005, pp. 676–696.
57
Dasgupta, Nabarun, Michele Jonsson Funk, Scott Proescholdbell, Annie Hirsch, and Kurst M.
Ribisl, “Cohort Study of the Impact of High-Dose Opioid Analgesics on Overdose
Mortality,” Pain Medicine, Vol. 17, No. 1, January 1, 2016, pp. 85–98.
Daubresse, Matthew, Hsien-Yen Chang, Yuping Yu, Shilpa Viswanathan, Nilay D. Shah,
Randall S. Stafford, Stefan P. Kruszewski, and G. Caleb Alexander, “Ambulatory Diagnosis
and Treatment of Nonmalignant Pain in the United States, 2000–2010,” Medical Care, Vol.
51, No. 10, October 2013, pp. 870–878.
Davis, Corey S., and Derek Carr, “Legal Changes to Increase Access to Naloxone for Opioid
Overdose Reversal in the United States,” Drug and Alcohol Dependence, Vol. 157,
December 1, 2015, pp. 112–120.
Davis, Corey, and Derek Carr, “State Legal Innovations to Encourage Naloxone Dispensing,”
Journal of the American Pharmacists Association, Vol. 57, No. 2, March–April 2017, pp.
S180–S184.
Davis, Corey S., Sarah Ruiz, Patrick Glynn, Gerald Picariello, and Alexander Y. Walley,
“Expanded Access to Naloxone Among Firefighters, Police Officers, and Emergency
Medical Technicians in Massachusetts,” American Journal of Public Health, Vol. 104, No. 8,
August 1, 2014, pp. e7–e9.
Davis, Corey S., Jessica K. Southwell, Virginia Radford Niehaus, Alexander Y. Walley, and
Michael W. Dailey, “Emergency Medical Services Naloxone Access: A National Systematic
Legal Review,” Academic Emergency Medicine, Vol. 21, No. 10, October 2014, pp. 1173–
1177.
DeHart, Dana D., and Cheri J. Shapiro, “Integrated Administrative Data and Criminal Justice
Research,” American Journal of Criminal Justice, Vol. 42, No. 2, July 2016, pp. 255–274.
Delcher, Chris, Alexander C. Wagenaar, Bruce A. Goldberger, Robert L. Cook, and Mildred M.
Maldonado-Molina, “Abrupt Decline in Oxycodone-Caused Mortality After Implementation
of Florida's Prescription Drug Monitoring Program,” Drug and Alcohol Dependence, Vol.
150, May 1, 2016, pp. 63–68.f
Deyo, Richard A., Sara E. Hallvik, Christi Hildebran, Miguel Marino, Eve Dexter, Jessica M.
Irvine, Nicole O’Kane, Joshua Van Otterloo, Dagan A. Wright, Gillian Leichtling, and Lisa
M. Millet, “Association Between Initial Opioid Prescribing Patterns and Subsequent Long-
Term Use Among Opioid-Naïve Patients: A Statewide Retrospective Cohort Study,” Journal
of General Internal Medicine, Vol. 32, No. 1, January 2017, pp. 21–27.
Dick, Andrew W., Rosalie Liccardo Pacula, Adam J. Gordon, Mark Sorbero, Rachel M. Burns,
Douglas L. Leslie, and Bradley D. Stein, “Growth in Buprenorphine Waivers for Physicians
Increased Potential Access to Opioid Agonist Treatment, 2002–11,” Health Affairs, Vol. 34,
No. 6, 2015, pp. 1028–1034.
58

Dokholyan, Rachel S., Lawrence H. Muhlbaier, John M. Falletta, Jeffrey P. Jacobs, David
Shahian, Constance K. Haan, and Eric D. Peterson, “Regulatory and Ethical Considerations
for Linking Clinical and Administrative Databases,” American Heart Journal, Vol. 157, No.
6, June 2009, pp. 971–982.
Doshi, Jalpa A., Franklin B. Hendrick, Jennifer S. Graff, and Bruce C. Stuart, “Data, Data
Everywhere, but Access Remains a Big Issue for Researchers: A Review of Access Policies
for Publicly Funded Patient-Level Health Care Data in the United States,” EGEMS (Wash
DC), Vol. 4, No. 2, 2016, p. 1204.
Dowell, Deborah, Tamara M. Haegerich, and Roger Chou, “CDC Guideline for Prescribing
Opioids for Chronic Pain—United States, 2016,” Journal of the American Medical
Association, Vol 65, No. 1, March 18, 2016, pp. 1624–1645.
Doyon, Suzanne, Carleigh Benton, Bruce A. Anderson, Michael Baier, Erin Haas, Lisa Hadley,
Jennifer Maehr, Kathleen Rebbert-Franklin, Yngvild Olsen, and Christopher Welsh,
“Incorporation of Poison Center Services in a State-Wide Overdose Education and Naloxone
Distribution Program,” American Journal on Addictions, Vol. 25, No. 4, June 2016, pp. 301–
306.
Dusetzina, Stacie B., Seth Tyree, and Anne-Marie Meyer, Linking Data for Health Services
Research: A Framework and Instructional Guide [Internet], webpage, Rockville, Md.:
Agency for Healthcare Resarch and Quality, September 4, 2014. As of July 1, 2018:
https://www.ncbi.nlm.nih.gov/books/NBK253312/
Dusetzina, Stacie B., Seth Tyree, Anne-Marie Meyer, Adrian Meyer, Laura Green, and William
R. Carpenter, Linking Data for Health Services Research: A Framework and Instructional
Guide, Rockville, Md.: Agency for Healthcare Research and Quality, report no. 14-EHC033-
EF, September 2014.
Dworsky, Michael, “Using All-Payer Claims Databases to Study Insurance and Health Care
Utilization Dynamic,” Journal of General Internal Medicine, Vol. 32, No. 10, October 2017,
pp. 1069–1070.
Estrada, Antonio L., “Health Disparities Among African-American and Hispanic Drug
Injectors—HIV, AIDS, Hepatitis B Virus and Hepatitis C Virus: A Review,” AIDS, Vol. 19,
Supplement 3, October 2005, pp. S47–S52.
Fairbairn, Nadia, Phillip O. Coffin, and Alexander Y. Walley, “Naloxone for Heroin,
Prescription Opioid, and Illicitly Made Fentanyl Overdoses: Challenges and Innovations
Responding to a Dynamic Epidemic,” Internal Journal of Drug Policy, Vol. 46, August
2017, pp. 172–179.
59
Feder, Kenneth A., Noa Krawczyk, and Brendan Saloner, “Medication-Assisted Treatment for
Adolescents in Specialty Treatment for Opioid Use Disorder,” Journal of Adolescent Health,
Vol. 60, No. 6, June 2017, pp. 747–750.
Fellegi, Ivan P., and Alan B. Sunter, “A Theory for Record Linkage,” Journal of the American
Statistical Association, Vol. 64, No. 328, 1969, pp. 1183–1210.
Finney, John W., Keith Humphreys, Daniel R. Kivlahan, and Alex H. S. Harris, “Why Health
Care Process Performance Measures Can Have Different Relationships to Outcomes for
Patients and Hospitals: Understanding the Ecological Fallacy,” American Journal of Public
Health, Vol. 101, No. 9, September 2011, pp. 1635–1642.
Friedman, Daniel J., Gibson Parrish, and David A. Ross, “Electronic Health Records and U.S.
Public Health: Current Realities and Future Promise,” American Journal of Public Health,
Vol. 103, No. 9, September 2013, pp. 1560–1567.
Garnick, Deborah W., Constance M. Horgan, Andrea Acevedo, Margaret T. Lee, Lee Panas,
Grant A. Ritter, Robert Dunigan, Alfred Bidorini, Kevin Campbell, Karin Haberlin, Alice
Huber, Dawn Lambert-Wacey, Tracy Leeper, Mark Reynolds, and David Wright, “Criminal
Justice Outcomes After Engagement in Outpatient Substance Abuse Treatment,” Journal of
Substance Abuse Treatment, Vol. 46, No. 3, March 2014, pp. 295–305.
Garnick, Deborah W., Constance M. Horgan, Andrea Acevedo, Frank McCorry, and Constance
Weisner, “Performance Measures for Substance Use Disorders--What Research Is Needed?”
Addiction Science and Clinical Practice, Vol. 7, No. 18, 2012.
Gilson, Aaron M., Scott M. Fishman, Barth L. Wilsey, Carlos Casamalhuapa, and Hassan Baxi,
“Time Series Analysis of California’s Prescription Monitoring Program: Impact on
Prescribing and Multiple Provider Episodes,” The Journal of Pain, Vol. 13, No. 2, February
2012, pp. 103–111.
Goerge, Robert, Leah Gjertson, and Ella De La Cruz, Administrative Data for the Public Good:
Opportunities for Advancing Evidence-Based Policymaking Using Data Held by the U.S.
Census Bureau, Chicago, Ill.: Chapin Hall at the University of Chicago, 2017.
Gordon, Adam J., Wei-Hsuan Lo-Ciganic, Gerald Cochran, Walid F. Gellad, Terri Cathers, and
Julie M. Donohue, “Treatment Quality for Buprenorphine Care: The Pot at the End of the
Rainbow [In Reply to ‘Measurement Quality of Buprenorphine Care’], Journal of Addiction
Medicine, Vol. 10, No. 3, May–June 2016, pp. 210–211.
Green, Carla A., Nancy A. Perrin, Shannon L. Janoff, Cynthia I. Campbell, Howard D. Chilcoat,
and Paul M. Coplan, “Assessing the Accuracy of Opioid Overdose and Poisoning Codes in
Diagnostic Information from Electronic Health Records, Claims Data, and Death Records,”
Pharmacoepidemiology and Drug Safety, Vol. 26, No. 5, May 2017, pp. 509–517.
60
Greenland, Sander, “A Review of Multilevel Theory for Ecologic Analyses,” Statistics in
Medicine, Vol. 21, No. 3, February 15, 2002, pp. 389–395.
Greenwood-Ericksen, Margaret B., Sabrina J. Poon, Lewis S. Nelson, Scott G. Weiner, and
Jeremiah D. Schuur, “Best Practices for Prescription Drug Monitoring Programs in the
Emergency Department Setting: Results of an Expert Panel,” Annals of Emergency Medicine,
Vol. 67, No. 6, June 2016, pp. 755–764.e4.
Gupta, Ravi, Nilay D. Shah, and Joseph S. Ross, “The Rising Price of Naloxone—Risks to
Efforts to Stem Overdose Deaths,” New England Journal of Medicine, Vol. 375, December
8, 2016, pp. 2213–2215.
Hadland, Scott E., J. Frank Wharam, and Mark A. Schuster “Trends in Receipt of Buprenorphine
and Naltrexone for Opioid Use Disorder Among Adolescents and Young Adults, 2001–
2014,” JAMA Pediatrics, Vol. 171, No. 8, August 1, 2017, pp. 747–755.
Haegerich, Tamara M., Leonard J. Paulozzi, Brian J. Manns, and Christopher M. Jones, “What
We Know, and Don’t Know, About the Impact of State Policy and Systems-Level
Interventions on Prescription Drug Overdose,” Drug and Alcohol Dependence, Vol. 145,
December 1, 2014, pp. 34–47.
Hall, H. Irene, Ruiguang Song, Philip Rhodes, Joseph Prejean, Qian An, Lisa M. Lee, John
Karon, Ron Brookmeyer, Edward H. Kaplan, Matthew T. McKenna, and Robert S. Janssen,
“Estimation of HIV Incidence in the United States,” Journal of the American Medical
Association, Vol. 300, No. 5, August 6, 2008, pp. 520–529.
Han, Huijun, Philip H. Kass, Barth L. Wilsey, and Chin-Shang Li, “Increasing Trends in
Schedule II Opioid Use and Doctor Shopping During 1999–2007 in California,”
Pharmacoepidemiology and Drug Safety, Vol. 23, No. 1, January 2014, pp. 26–35.
Harle, Christopher A., Robert L. Cook, Heidi S. Kinsell, and Jeffrey S. Harman, “Opioid
Prescribing by Physicians with and Without Electronic Health Records,” Journal of Medical
Systems, Vol. 38, No. 11, November 2014, p. 138.
Hartung, Daniel M., Sharia M. Ahmed, Luke Middleton, Joshua Van Otterloo, Kun Zhang,
Shellie Keast, Hyunjee Kim, Kirbee Johnston, and Richard A. Deyo, “Using Prescription
Monitoring Program Data o Characterize Out!Oof!Pocket Payments for Opioid Prescriptions
in a State Medicaid Program,” Pharmacoepidemiology and Drug Safety, Vol. 26, No. 9,
September 2017, pp. 1053–1060.
Häyrinen, Kristiina, Kaija Saranto, and Pirkko Nykänen, “Definition, Structure, Content, Use
and Impacts of Electronic Health Records: A Review of the Research Literature,”
International Journal of Medical Informatics, Vol. 77, No. 5, May 2008, pp. 291–304.
61

Healthcare Cost and Utilization Project, “HCUP Fast Stats—Opioid-Related Hospital Use,”
webpage, June 2018. As of July 1, 2018:
https://www.hcup-us.ahrq.gov/faststats/OpioidUseServlet
Health Resources and Services Administration, “Health Center Patient Survey,” webpage,
undated. As of May 30, 2018:
https://bphc.hrsa.gov/datareporting/research/hcpsurvey/index.html
Health Resources and Services Administration, “Uniform Data System (UDS) Resources,”
webpage, June 2018. As of May 17, 2018:
https://bphc.hrsa.gov/datareporting/reporting/index.html
HHS—See U.S. Department of Health and Human Services.
Hirsch, Anne, Scott K. Proescholdbell, William Bronson, and Nabarun Dasgupta, “Prescription
Histories and Dose Strengths Associated with Overdose Deaths,” Pain Medicine, Vol. 15,
No. 7, July 2014, pp. 1187–1195.
Houry, Debra, “Testimony from Debra Houry, M.D. on Fentanyl: The Next Wave of the Opioid
Crisis before Committee on Energy and Commerce,” testimony before the Committee on
Energy and Commerce, Subcommittee on Oversight and Investigations, Washington, D.C.,
March 21, 2017. As of July 1, 2018:
https://www.hhs.gov/about/agencies/asl/testimony/2017-03/fentanyl-next-wave-opioid-
crisis.html
Hser, Yih-Ing, Larissa J. Mooney, Andrew J. Saxon, Karen Miotto, Douglas S. Bell, and David
Huang, “Chronic Pain Among Patients with Opioid Use Disorder: Results from Electronic
Health Records Data,” Journal of Substance Abuse Treatment, Vol. 77, June 2017, pp. 26–
30.
HRSA—See Health Resources and Services Administration.
Hudson, Toni-Marie, Benjamin G. Klekamp, and Sarah D. Matthews, “Local Public Health
Surveillance of Heroin-Related Morbidity and Mortality, Orange County, Florida, 2010–
2014,” Public Health Reports, Vol. 132, Supplement 1, July–August 2017, pp. 80S–87S.
Interagency Pain Research Coordinating Committee, National Pain Strategy: A Comprehensive
Population Health-Level Strategy for Pain, Washington, D.C.: Department of Health and
Human Services, 2015. As of June 27, 2018:
https://iprcc.nih.gov/docs/HHSNational_Pain_Strategy.pdf
Ising, Amy, Scott Proescholdbell, Katherine J. Harmon, Nidhi Sachdeva, Stephen W. Marshall,
and Anna E. Waller, “Use of Syndromic Surveillance Data to Monitor Poisonings and Drug
Overdoses in State and Local Public Health Agencies,” Injury Prevention, Vol. 22,
Supplement 1, 2016, pp. i43–i49.
62
Johnson, Kristen M., Meghan Fibbi, Debra Langer, Karol Silva, and Stephen E. Lankenau,
“Prescription Drug Misuse and Risk Behaviors Among Young Injection Drug Users,”
Journal of Psychoactive Drugs, Vol. 45, No. 2, April–June 2013, pp. 112–121.
Jones, Christopher M., Melinda Campopiano, Grant Baldwin, and Elinore McCance-Katz,
“National and State Treatment Need and Capacity for Opioid Agonist Medication-Assisted
Treatment,” American Journal of Public Health, Vol. 105, No. 8, August 2015, pp. e55–e63.
Kalyanam, Janani, Takeo Katsuki, Gert R. G. Lanckriet, and Tim K. Mackey, “Exploring Trends
of Nonmedical Use of Prescription Drugs and Polydrug Abuse in the Twittersphere Using
Unsupervised Machine Learning,” Addictive Behaviors, Vol. 65, February 2017, pp. 289–
295.
Kalyanam, Janani, and Tim K. Mackey, “A Review of Digital Surveillance Methods and
Approaches to Combat Prescription Drug Abuse,” Current Addiciton Reports, Vol. 4, No. 4,
December 2017, pp. 1–13.
Katsuki, Takeo, Tim Ken Mackey, and Raphael Cuomo, “Establishing a Link Between
Prescription Drug Abuse and Illicit Online Pharmacies: Analysis of Twitter Data,” Journal of
Medical Internet Research, Vol. 17, No. 12, December 16, 2015, p. e280.
Katz, Nathaniel, Lee Panas, MeeLee Kim, Adele D. Audet, Arnold Bilansky, John Eadie, Peter
Kreiner, Florence C. Paillard, Cindy Thomas, and Grant Carrow, “Usefulness of Prescription
Monitoring Programs for Surveillance—Analysis of Schedule II Opioid Prescription Data in
Massachusetts, 1996–2006,” Pharmacoepidemiology and Drug Safety, Vol. 19, No. 2,
February 2010, pp. 115–123.
Kennedy-Hendricks, Alene, Matthew Richey, Emma E. McGinty, Elizabeth A. Stuart, Colleen
L. Barry, and Daniel W. Webster, “Opioid Overdose Deaths and Florida’s Crackdown on Pill
Mills,” American Journal of Public Health, Vol. 106, No. 2, February 2016, pp. 291–297.
Kho, Abel N., John P. Cashy, Kathryn L. Jackson, Adam R. Pah, Satyender Goel, Jörn Boehnke,
John Eric Humphries, Scott Duke Kominers, Bala N. Hota, Shannon A. Sims, Bradley A.
Malin, Dustin D. French, Theresa L. Walunas, David O. Meltzer, Erin O. Kaleba, Roderick
C. Jones, and William L. Galanter, “Design and Implementation of a Privacy Preserving
Electronic Health Record Linkage Tool in Chicago,” Journal of the American Medical
Informatics Association, Vol. 22, No. 5, September 2015, pp. 1072–1080.
Knudsen, Hannah K., Michelle R. Lofwall, Jennifer R. Havens, and Sharon L. Walsh, “States’
Implementation of the Affordable Care Act and the Supply of Physicians Waivered to
Prescribe Buprenorphine for Opioid Dependence,” Drug and Alcohol Dependence, Vol. 157,
December 1, 2015, pp. 36–43.
Krebs, Erin E., Amy Gravely, Sean Nugent, Agnes C. Jensen, Beth DeRonne, Elizabeth S.
Goldsmith, Kurt Kroenke, Matthew J. Bair, and Siamak Noorbaloochi, “Effect of Opioid vs.
63

Nonopioid Medications on Pain-Related Function in Patients with Chronic Back Pain or Hip
or Knee Osteoarthritis Pain: The SPACE Randomized Clinical Trial,” Journal of the
American Medical Association, Vol. 319, No. 9, March 6, 2018, pp. 872–882.
Krebs, Erin E., Jon D. Lurie, Gilbert Fanciullo, Tor D. Tosteson, Emily A. Blood, Timothy S.
Carey, and James N. Weinstein, “Predictors of Long-Term Opioid Use Among Patients with
Painful Lumbar Spine Conditions,” Journal of Pain, Vol. 11, No. 1, January 2010, pp. 44–
52.
Kreiner, Peter W., Gail K. Strickler, Eduardo A. Undurraga, Maria E. Torres, Ruslan V. Nikitin,
and Anne Rogers, “Validation of Prescriber Risk Indicators Obtained from Prescription Drug
Monitoring Program Data,” Drug and Alcohol Dependence, Vol. 173, Supplement 1, April 1,
2017, pp. S31–S38.
Kum, Hye-Chung, Ashok Krishnamurthy, Ashwin Machanavajjhala, Michael K. Reiter, and
Stanley Ahalt, “Privacy Preserving Interactive Record Linkage (PPIRL),” Journal of the
American Medical Informatics Association, Vol. 21, No. 2, March–April 2014, pp. 212–220.
Kwiatkowski, Carol F., Robert E. Booth, Laura V. Lloyd, “The Effects of Offering Free
Treatment to Street-Recruited Opioid Injectors,” Addiction, Vol. 95, No. 5, May 2000, pp.
697–704.
Ladegaard, Isak, “Instantly Hooked? Freebies and Samples of Opioids, Cannabis, MDMA, and
Other Drugs in an Illicit E-Commerce Market,” Journal of Drug Issues, Vol. 48, No. 2,
December 2017.
Lin, Dora H., Eleanor Lucas, Irene B. Murimi, Katherine Jackson, Michael Baier, Shannon
Frattaroli, Andrea C. Gielen, Patience Moyo, Linda Simoni-Wastila, and G. Caleb
Alexander, “Physician Attitudes and Experiences with Maryland's Prescription Drug
Monitoring Program (PDMP),” Addiction, Vol. 112, No. 2, February 2017, pp. 311–319.
Lingren, Todd, Senthilkumar Sadhasivam, Xue Zhang, and Keith Marsolo, “Electronic Medical
Records as a Replacement for Prospective Research Data Collection in Postoperative Pain
and Opioid Response Studies,” International Journal of Medical Informatics, Vol. 111,
March 2018, pp. 45–50.
Lyons, B. Casey, and Kirsten Madison, Predictive Risk Evaluation to Combat Overdose Grant
(PRECOG),” Maryland Department of Health and Mental Hygiene, March 31, 2017. As of
September 18, 2017:
http://www.pdmpassist.org/pdf/31-A-4_Maryland.pdf
Madden, Jeanne M., Matthew D. Lakoma, Donna Rusinak, Christine Y. Lu, and Stephen B.
Soumerai, “Missing Clinical and Behavioral Health Data in a Large Electronic Health
Record (EHR) System,” Journal of American Medical Informatics Association, Vol. 23, No.
6, November 2016, pp. 1143–1149.
64

Maddux, James F., and David P. Desmond, “Outcomes of Methadone Maintenance 1 Year After
Admission,” Journal of Drug Issues, Vol. 27, No. 2, April 1, 1997, pp. 225–238.
Mai, Jaymie, Gary Franklin, and David Tauben, “Guideline for Prescribing Opioids to Treat Pain
in Injured Workers,” Physical Medicine and Rehabilation Clinics of North America, Vol. 26,
No. 3, August 2015, pp. 453–465.
Manasco, A. Travis, Christopher Griggs, Rebecca Leeds, Breanne K. Langlois, Alan H. Breaud,
Patricia M. Mitchell, and Scott G. Weiner, “Characteristics of State Prescription Drug
Monitoring Programs: A State-by-State Survey,” Pharmacoepidemiology and Drug Safety,
Vol. 25, No. 7, July 2016, pp. 847–851.
McCormick, Meghan, Jennifer Koziol, and Kelly Sanchez, “Development and Use of a New
Opioid Overdose Surveillance System, 2016,” Substance Abuse, No. 1091, 2017, pp. 71–76.
McCoy, Allison B., Adam Wright, Michael G. Kahn , Jason S. Shapiro, Elmer Victor Bernstam,
and Dean F. Sittig, “Matching Identifiers in Electronic Health Records: Implications for
Duplicate Records and Patient Safety,” BMJ Quality and Safety, Vol. 22, No. 3, March 2013,
pp. 219–224.
Méray, Nora, Johannes B. Reitsma, Anita C. J. Ravelli, and Gouke J. Bonsel, “Probabilistic
Record Linkage Is a Valid and Transparent Tool to Combine Databases Without a Patient
Identification Number,” Journal of Clinical Epidemiology, Vol. 60, No. 9, September 2007,
pp. 883–891.
Merlin, Mark A., Navin Ariyaprakai, and Faizan H. Arshad, “Assessment of the Safety and Ease
of Use of the Naloxone Auto-Injector for the Reversal of Opioid Overdose,” Open Access
Emergency Medicine, Vol. 7, June 8, 2015, pp. 21–24.
Minnesota Department of Health, “Opioid Dashboard,” webpage, undated. As of June 27, 2018:
http://www.health.state.mn.us/divs/healthimprovement/opioid-dashboard
Morgan, Jake R., Bruce R. Schackman, Jared A. Leff, Benjamin P. Linas, and Alexander Y.
Walley, “Injectable Naltrexone, Oral Naltrexone, and Buprenorphine Utilization and
Discontinuation Among Individuals Treated for Opioid Use Disorder in a United States
Commercially Insured Population,” Journal of Substance Abuse Treatment, Vol. 85,
February 2018, pp. 90–86.
Murray, Michael D., “Use of Data from Electronic Health Records for Pharmacoepidemiology,”
Current Epidemiology Reports, Vol. 1, No. 4, December 2014, pp. 186–193.
National Institute on Drug Abuse, “Overdose Death Rates,” webpage, revised September 2017.
As of July 1, 2018:
https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates
65

National Institute of Justice, “NIJ’s Drugs and Crime Research: Arrestee Drug Abuse
Monitoring Programs,” webpage, June 18, 2014. As of July 6, 2018:
https://www.nij.gov/topics/drugs/markets/adam/pages/welcome.aspx
NEMSIS, “Public Naloxone Administration Dashboard,” webpage, undated. As of May 17,
2018:
https://nemsis.org/view-reports/public-reports/version-3-public-dashboards/public-naloxone-
administration-dashboard
Nuckols, Teryl K., Laura Anderson, Ioana Popescu, Allison L. Diamant, Brian Doyle, Paul Di
Capua, and Roger Chou, “Opioid Prescribing: ASystematic Review and Critical Appraisal of
Guidelines for Chronic Pain,” Annals of Internal Medicine, Vol. 160, No. 1, January 2014,
pp. 38–47.
OCHIN, “ADVANCE: Accelerating Data Value Across a National Community Health Center
Network,” webpage, March 1, 2014–August 1, 2018. As of May 17, 2018:
https://ochin.org/ochin-research/advance-research-summary
O’Kane, Nicole, Sara E. Hallvik, Miguel Marino, Joshua Van Otterloo, Christi Hildebran,
Gillian Leichtling, and Richard A. Deyo, “Preparing a Prescription Drug Monitoring
Program Data Set for Research Purposes,” Pharmacoepidemiology and Drug Safety, Vol. 26,
No. 9, September 2016, pp. 993–997.
“Opioid Mapping Initiative,” webpage, undated. As of May 17, 2018:
http://opioidmappinginitiative-opioidepidemic.opendata.arcgis.com
Pardo, Bryce, “Do More Robust Prescription Drug Monitoring Programs Reduce Prescription
Opioid Overdose?” Addiction, Vol. 112, No. 10, October 2017, pp. 1773–1783.
Parker, Jason, Courtney Cuthbertson, Scott Loveridge, Mark Skidmore, and Will Dyar,
“Forecasting State-Level Premature Deaths from Alcohol, Drugs, and Suicides Using Google
Trends Data,” Journal of Affective Disorders, Vol. 213, April 15, 2017, pp. 9–15.
Paulozzi, Leonard J., Edwin M. Kilbourne, and Hema A. Desai, “Prescription Drug Monitoring
Programs and Death Rates from Drug Overdose,” Pain Medicine, Vol. 12, No. 5, May 2011,
pp. 747–754.
pcornet, “Partner Networks,” webpage, undated. As of May 17, 2018:
http://www.pcornet.org/participating-networks
PDAPS—See Prescription Drug Abuse Policy System.
PDMP—See Prescription Drug Monitoring Program.
Piper, Brian J., Claire E. Desrosiers, John W. Lipovsky, Matthew A. Rodney, Robert P. Baker,
Kenneth L. McCall, Stephanie D. Nichols, and Sarah L. Martin, “Use and Misuse of Opioids
in Maine: Results From Pharmacists, the Prescription Monitoring, and the Diversion Alert
66

Programs,” Journal of Studies on Alcohol and Drugs, Vol. 77, No. 4, July 2016, pp. 556–
565.
Porucznik, Christina A., Erin M. Johnson, Robert T. Rolfs, and Brian C. Sauer, “Specialty of
Prescribers Associated with Prescription Opioid Fatalities in Utah, 2002–2010,” Pain
Medicine, Vol. 15, No. 1, January 1, 2014, pp. 73–78.
Prescription Drug Abuse Policy System, homepage, undated. As of May 17, 2018:
http://www.pdaps.org
Prescription Drug Monitoring Program Center of Excellence at Brandeis, Using PDMPs to
Improve Medical Care: Washington State’s Data Sharing Initiative with Medicaid and
Workers’ Compensation, Notes from the Field series, April 2013. As of June 27, 2018:
http://www.pdmpassist.org/pdf/COE_documents/Add_to_TTAC/washington_nff_final.pdf
Prescription Drug Monitoring Program, Training and Technical Assistance Center, “Status of
Prescription Drug Monitoring Programs (PDMPs),” webpage, August 24, 2017. As of
September 18, 2017:
http://www.pdmpassist.org/pdf/PDMP_Program_Status_20170824.pdf
Prescription Drug Monitoring Program, “Release of PDMP Data for Research, Epidemiological,
or Educational Purposes,” webpage, December 5, 2017. As of May 17, 2018:
http://www.pdmpassist.org/pdf/Data_Use_Res_Epi_Educ_20171205.pdf
Price, Thomas E., “Secretary Price Announces HHS Strategy for Fighting Opioid Crisis,”
Atlanta, Ga., National Rx Drug Abuse and Heroin Summit, April 19, 2017. As of June 27,
2018:
https://www.hhs.gov/about/leadership/secretary/speeches/2017-speeches/secretary-price-
announces-hhs-strategy-for-fighting-opioid-crisis/index.html
Quast, Troy, Eric A. Storch, and Svetlana Yampolskaya, “Opioid Prescription Rates and Child
Removals: Evidence from Florida,” Health Affairs, Vol. 37, No. 1, January 2018, pp. 134–
139.
Raghupathi, Wullianallur, and Viju Raghupathi, “Big Data Analytics in Healthcare: Promise and
Potential,” Health Information Science and Systems, Vol. 2, No. 3, 2014.
Rhode Island Department of Health, “Policy: Identifying and Reporting Confirmed Accidental
Drug-Related Overdose Deaths, June 2015,” 2015. As of September 21, 2017:
http://www.health.ri.gov/publications/policies/IdentifyingAndReportingConfirmedAccidenta
lDrugRelatedOverdoseDeaths.pdf
Rinaldo, Suzanne Gelber, and David W. Rinaldo, “Report I: Availability Without Accessability?
State Medicaid Coverage and Authorization Requirements for Opioid Dependence
Medications: Implications for Opioid Addiction Treatment,” in Advancing Access to
67
Addiction Medications: Implications for Opioid Addiction Treatment, Chevy Chase, Md.:
American Society of Addiction Medicine, 2013.
Ringwalt, Christopher, Sharon Schiro, Meghan Shanahan, Scott Proescholdbell, Harold Meder,
Anna Austin, and Nidhi Sachdeva, “The Use of a Prescription Drug Monitoring Program to
Develop Algorithms to Identify Providers with Unusual Prescribing Practices for Controlled
Substances,” Journal of Primary Prevention, Vol. 36, No. 5, October 2015, pp. 287–299.
Robert, Andrew W., Joel F. Farley, G. Mark Holmes, Christine U. Oramasionwu, Chris
Ringwalt, Betsy Sleath, and Asheley C. Skinner, “Controlled Substance Lock-In Programs:
Examining An Unintended Consequence Of A Prescription Drug Abuse Policy,” Health
Affairs, Vol. 35, No. 10, October 1, 2016, pp. 1884–1892.
Robinson, W. S., “Ecological Correlations and the Behavior of Individuals,” American
Sociological Review, Vol. 15, No. 3, June 1950, pp. 351–357.
Rosenblatt, Roger A., C. Holly A. Andrilla, Mary Catlin, and Eric H. Larson, “Geographic and
Specialty Distribution of U.S. Physicians Trained to Treat Opioid Use Disorder,” Annals of
Family Medicine, Vol. 13, No. 1, January–February 2015, pp. 23–26.
Ross, Joseph S., and Harlan M. Krumholz, “Ushering in a New Era of Open Science Through
Data Sharing: The Wall Must Come Down,” Journal of the American Medical Association,
Vol. 309, No. 13, April 3, 2013, pp. 1355–1356.
Rudd, Rose A., Puja Seth, Felicita David, and Lawrence Scholl, “Increases in Drug and Opioid-
Involved Overdose Deaths—United States, 2010–2015,” Morbidity and Mortality Weekly
Report (MMWR), Vol. 65, No. 50–51, December 2016, pp. 1445–1452.
Ruhm, Christopher J., “Geographic Variation in Opioid and Heroin Involved Drug Poisoning
Mortality Rates,” American Journal of Preventive Medicine, Vol. 53, No. 6, August 1, 2017,
pp. 745–753
Russo, Elise, Dean F. Sittig, Daniel R. Murphy, and Hardeep Singh, “Challenges in Patient
Safety Improvement Research in the Era of Electronic Health Records,” Healthcare, Vol. 4,
No. 4, December 2016, pp. 285–290.
Saloner, Brendan, “Using Data Science to Identify Individuals at High Risk of Opioid Overdose:
A Multiyear Data Linkage Project in Maryland,” paper presented at the Association for
Public Policy Analysis and Management 38th Annual Fall Research Conference: The Role of
Research in Making Government More Effective, Washington, D.C., November 4, 2016.
Saloner, Brendan, and Shankar Karthikeyan, “Changes in Substance Abuse Treatment Use
Among Individuals with Opioid Use Disorders in the United States, 2004–2013,” Journal of
the American Medical Association, Vol. 314, No. 14, October 13, 2015, pp. 1515–1517.
68

Sayers, Adrian, Yoav Ben-Shlomo, Ashley W. Blom, and Fiona Steele, “Probabilistic Record
Linkage,” Internal Journal of Epidemiology, Vol. 45, No. 3, June 2016, pp. 954–964.
Schuur, Jeremiah D., Akash Shah, Zheyang Wu, Howard P. Forman, and Cary P. Gross, “The
Impact of Medicaid Coverage and Reimbursement on Access to Diagnostic Mammography,”
Cancer, Vol. 115, No. 23, December 1, 2009, pp. 5566–5578.
Shen, Yu-Chu, and Stephen Zuckerman, “The Effect of Medicaid Payment Generosity on Access
and Use Among Beneficiaries,” Health Services Research, Vol. 40, No. 3, June 2005, pp.
723–744.
Slavova, Svetla, Julia F.Costich, Terry L.Bunn, Huong Luu, Michael Singleton, Sarah L.
Hargrove, Jeremy S. Triplett, Dana Quesinberry, William Ralston, and Van Ingram, “Heroin
and Fentanyl Overdoses in Kentucky: Epidemiology and Surveillance,” International
Journal of Drug Policy, Vol. 46, August 2017, pp. 120–129.
Stein, Bradley D., Adam J. Gordon, Andrew W. Dick, Rachel M. Burns, Rosalie Liccardo
Pacula, Carrie M. Farmer, Douglas L. Leslie, and Mark Sobero, “Supply of Buprenorphine
Waivered Physicians: The Influence of State Policies,” Journal of Substance Abuse
Treatment, Vol. 48, No. 1, January 2015, pp. 104–111.
Stein, Bradley D., Rosalie Liccardo Pacula, Adam J. Gordon, Rachel M. Burns, Douglas L.
Leslie, Mark J. Sorbero, Sebastian Bauhoff, Todd W. Mandell, and Andrew W. Dick,
“Where Is Buprenorphine Dispensed to Treat Opioid Use Disorders? The Role of Private
Offices, Opioid Treatment Programs, and Substance Abuse Treatment Facilities in Urban and
Rural Counties,” The Milbank Quarterly, Vol. 93, No. 3, September 2015, pp. 561–583.
Substance Abuse and Mental Health Services Administration, “DAWN: Drug Abuse Warning
Network,” webpage, 2018,. As of July 16, 2018:
https://www.samhsa.gov/data/data-we-collect/dawn-drug-abuse-warning-network
Tennessee Department of Health, “Data Dashboard,” webpage, undated. As of May 17, 2018:
https://www.tn.gov/health/health-program-areas/pdo/pdo/data-dashboard.html
Thomas, Cindy Parks, Deborah W. Garnick, Constance M. Horgan, Frank McCorry, Amanda
Gmyrek, Mady Chalk, David R. Gastfriend, Suzanne Gelber Rinaldo, Joann Albright, Victor
A. Capoccia, Alex H. S. Harris, Henrick J. Harwood, Pamela Greenberg, Tami L. Mark,
Huong Un, Marla Oros, Mark Stringer, and James Thatcher, “Advancing Performance
Measures for Use of Medications in Substance Abuse Treatment,” Journal of Substance
Abuse Treatment, Vol. 40, No. 1, January 2011, pp. 35–43.
Tromp, Miranda, Anita C. J. Ravelli, Gouke J. Bonsel, Arie Hasman, and Johannes B. Reitsma,
“Results from Simulated Data Sets: Probabilistic Record Linkage Outperforms Deterministic
Record Linkage,” Journal of Clinical Epidemiology, Vol. 54, No. 5, May 2011, pp. 565–572.
69

U.S. Census Bureau, “Federal Statistical Research Data Centers,” webpage, last revised January
14, 2015. As of May 17, 2018:
https://www.census.gov/about/adrm/fsrdc/about.html
U.S. Census Bureau, “United States Census Bureau Data Repository,” webpage, 2017. As of
May 30, 2018:
https://census.icpsr.umich.edu/census
U.S. Department of Health and Human Services, “Help, Resources, and Information: National
Opioid Crisis,” webpage, undated. As of March 28, 2018:
https://www.hhs.gov/opioids
U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services
Administration, Center for Behavioral Health Statistics and Quality, Drug Abuse Warning
Network, 2011: National Estimates of Drug-Related Emergency Department Visits,
Rockville, Md., May 2013.
U.S. Department of Health and Human Services, Behavioral Health Coordinating Committee,
Prescription Drug Abuse Subcommittee, Addressing Prescription Drug Abuse in the United
States: Current Activities and Future Opportunities, Washington, D.C., September 2013. As
of September 18, 2017:
http://www.cdc.gov/drugoverdose/pdf/hhs_prescription_drug_abuse_report_09.2013.pdf
U.S. Supreme Court, Gobeille v. Liberty Mutual Insurance Co. 577 U.S., 2016. As of June 11,
2018:
https://supreme.justia.com/cases/federal/us/577/14-181
Wakeland, Wayne, Alexandra Nielsen, and Peter Geissert, “Dynamic Model of Nonmedical
Opioid Use Trajectories and Potential Policy Interventions,” American Journal of Drug and
Alcohol Abuse, Vol. 41, No. 6, November 2015, pp. 508–518.
Walley, Alexander Y., Ziming Xuan, H. Holly Hackman, Emily Quinn, Maya Doe-Simkins,
Amy Sorensen-Alawad, Sarah Ruiz, and Al Ozonoff, “Opiod Overdose Rates and
Implementation of Overdose Education and Nasal Naloxone Distribution in Massachusetts:
Interrupted Time Series Analysis,” BMJ, Vol. 346, 2013.
Warner, Margaret, Leonard J. Paulozzi, Kurt B. Nolte, Gregory G. Davis, and Lewis S. Nelson,
“State Variation in Certifying Manner of Death and Drugs Involved in Drug Intoxication
Deaths,” Academic Forensic Pathology, Vol. 3, No. 2, June 2013, pp. 231–237.
Watkins, Katherine E., Allison J. Ober, Karen Lamp, Mimi Lind, Claude Setodji, Karen Chan
Osilla, Sarah B. Hunter, Colleen M. McCullough, Kirsten Becker, Praise O. Iyiewuare,
Allison Diamant, Keith Heinzerling, and Harold Alan Pincus, “Collaborative Care for Opioid
and Alcohol Use Disorders in Primary Care: The SUMMIT Randomized Clinical Trial,”
JAMA Internal Medicine, Vol. 177, No. 10, August 28, 2017, pp. 1480–1488.
70
Weiskopf, Nicole Gray, and Chunhua Weng, “Methods and Dimensions of Electronic Health
Record Data Quality Assessment: Enabling Reuse for Clinical Research,” Journal of
American Medical Informatics Association, Vol. 20, January 1, 2013, pp. 144–151.
Weiner, Scott G., Olesya Baker, Sabrina J. Poon, Ann F. Rodgers, Chad Garner, Lewis S.
Nelson, and Jeremiah D. Schuur, “The Effect of Opioid Prescribing Guidelines on
Prescriptions by Emergency Physicians in Ohio,” Annals of Emergency Medicine, Vol. 70,
No. 6, December 2017, pp. 799–808.
Wheeler, Eliza, T. Stephen Jones, Michael K. Gilbert, and Peter J. Davidson, “Opioid Overdose
Prevention Programs Providing Naloxone to Laypersons—United States, 2014,” Morbidity
and Mortality Weekly Report (MMWR), Vol. 64, No. 23, June 19, 2015, pp. 631–635.
Winkler, William E., The State of Record Linkage and Current Research Problems, Washington,
D.C.: Statistical Research Division, U.S. Census Bureau, 1999.
Winkler, William E., Overview of Record Linkage and Current Research Directions,
Washington, D.C.: Statistical Research Division, U.S. Census Bureau, research report series
(stiastics #2006-2), February 8, 2006.
Wilsey, Barth L., Scott M. Fishman, Aaron M. Gilson, Carlos Masamalhuapa, Hassan Baxi, Tzu-
Chun Lin, and Chin-Shang Li, “An Analysis of the Number of Multiple Prescribers for
Opioids Utilizing Data from the California Prescription Monitoring Program*,”
Pharmacoepidemiology and Drug Safety, Vol. 20, No. 12, December 2011, pp. 1262–1268.
Wright, Elizabeth A., Jeffrey N. Katz, Stanley Abrams, Daniel H. Solomon, and Elena Losina,
“Trends in Prescription of Opioids from 2003–2009 in Persons with Knee Osteoarthritis,”
Arthritis Care and Research, Vol. 66, No. 10, October 2014, pp. 1489–1495.
Van Hout, Marie Claire, and Evelyn Hearne, “New Psychoactive Substances (NPS) on
Cryptomarket Fora: An Exploratory Study of Characteristics of Forum Activity Between
NPS Buyers and Vendors,” Internal Journal of Drug Policy, Vol. 40, February 2017, pp.
102–110.
Volkow, Nora D., and Francis S. Collins, “The Role of Science in Addressing the Opioid Crisis,”
New England Journal of Medicine, Vol. 377, No. 4, July 27, 2017, pp. 391–394.
Volkow, Nora D., Thomas R. Frieden, Pamela S. Hyde, and Stephen S. Cha, “Medication-
Assisted Therapies—Tackling the Opioid-Overdose Epidemic,” New England Journal of
Medicine, Vol. 370, No. 22, May 29, 2014, pp. 2063–2066.
Yoo, Byung-Kwang, Andrea Berry, Megumi Kasajima, and Peter G. Szilagyi, “Association
Between Medicaid Reimbursement and Child Influenza Vaccination Rates,” Pediatrics, Vol.
126, No. 5, November 2010, pp. e998–e1010.
71

72
Appendix—Overview of Types of Secondary Data Sources and
Data Inventory Content
73
Appendix Table of Contents
Overview of Types of Secondary Data Sources and Data Inventory Content ...................................................................................................................74
Table A.1. National Survey Data ......................................................................................................................................................................................75
Table A.2. Claims and Electronic Health Records Secondary Data Sources ....................................................................................................................78
Table A.3. Mortality Records ............................................................................................................................................................................................84
Table A.4. Prescription Monitoring Secondary Data Sources ...........................................................................................................................................86
Table A.5. Contextual and Policy Data Sources ................................................................................................................................................................89
Table A.6. Other National, State, and Local Secondary Data Sources ..............................................................................................................................91
Data Description Summary Examples of Important Measures Data Source Examples
Information on Linking
Capability
National surveys Description: Generally household or school-based surveys with self- Prescription opioid use, heroin use, National Survey on Drug State, substate, and
(Table A.1) reported information on drug use and health; other surveys are of
hospitals, treatment facilities, or of other medical service providers
Geographic coverage: National
Timing: Generally collected and available annually
opioid use disorders, medical
conditions, health care utilization
Use and Health, National
Ambulatory Medical Care
Survey, National Survey of
Substance Abuse
Treatment Services Data
person-level linkages
possible. See Table A.1
for details.
Electronic health Description: An EHR is a digital version of a patient’s paper chart. Previously prescribed opioids or Stanford Translational State, substate, and
records (EHRs) While an EHR contains the medical and treatment histories of other medications; patient history, Research Integrated person-level linkages
(Table A.2) patients, an EHR system is built to go beyond standard clinical data
collected in a provider’s office and can be inclusive of a broader view
of a patient’s care. EHRs contain
a patient’s medical history,
diagnoses, medications, treatment plans, allergies, radiology images,
laboratory, and test results.
Geographic coverage: Varies by source
Timing: Near-real time or real-time collection
medications, clinical conditions,
treatment plans, and lab/test
results; may include clinician notes
Database, HealthCore
Integrated Research
Database, Group Health
Cooperative in
Washington State
possible. See Table A.2
for details.
Claims data Description: Patient-level claims data for reimbursement for services Prescription drug utilization; service IMS, Symphony Health, State, substate, and
(Table A.2) submitted by health care providers and pharmacies to insurance
companies. Validated algorithms to identify opioid misuse or abuse
from claims data are being developed.
Geographic coverage: Varies by source
Timing: Varies by source
utilization Truven Marketscan data,
Medicaid claims, Medicare
Part D Prescription Drug
Event data
person-level linkages
possible. See Table A.2
for details.
Mortality Description: Death rates and causes of death by drug compound Rates of opioid-involved deaths, Centers for Disease State, substate, and
records and/or International Classification of Diseases (ICD) code. Additional drugs involved in overdose deaths Control and Prevention person-level linkages
(Table A.3) information can include toxicology reports.
Geographic coverage: National or single state
Timing: Generally available annually
(CDC) WONDER Multiple
Cause of Death data, Fatal
Accident Reporting
System, National Death
Index (NDI)
possible. See Table A.3
for details.
Prescription Description: Data systems to track and monitor the distribution or Opioid prescribing rates (by type), Automation of Reports State, substate, and
monitoring data prescription of controlled substances indicators of "doctor shopping," and Consolidated Orders person-level linkages
(Table A.4) Geographic coverage: Varies by source
Timing: Varies by source
geographic variation in opioid
distribution
System; state prescription
drug-monitoring programs
possible. See Table A.4
for details.
Contextual and Description: Causal analyses of the effects of policy changes on State opioid policies, state and Area Health Resource Typically merged at the
policy data opioid-related outcomes generally use data on state laws from these county demographic and Files, Policy Surveillance state or county level
(Table A.5) sources and/or includes controls for state or county characteristics to
support causal interpretation.
Geographic coverage: National
Timing: Varies, but generally semiannually
socioeconomic factors, state and
county health care variables
System, Prescription Drug
Abuse Policy System
with other data on
opioid-related
outcomes
Other national, Description: Includes data collected through law enforcement, Law enforcement drug seizures, NEMSIS, National Poison State, substate, and
state, and local national public health surveillance systems (e.g., poison control nonfatal opioid overdose, opioid- Data System, Healthcare person-level linkages
sources centers, emergency department visits), overdose education and related emergency department Cost and Utilization possible. See Table A.6
(Table A.6) naloxone distribution programs, and hospitalization and emergency
departments
Geographic coverage: Varies by source
Timing: Varies by source
visits and hospitalizations, naloxone
distribution through community
organizations
Project (HCUP) emergency
department and
hospitalization data
for details.
74
Overview of Types of Secondary Data Sources and Data Inventory Content
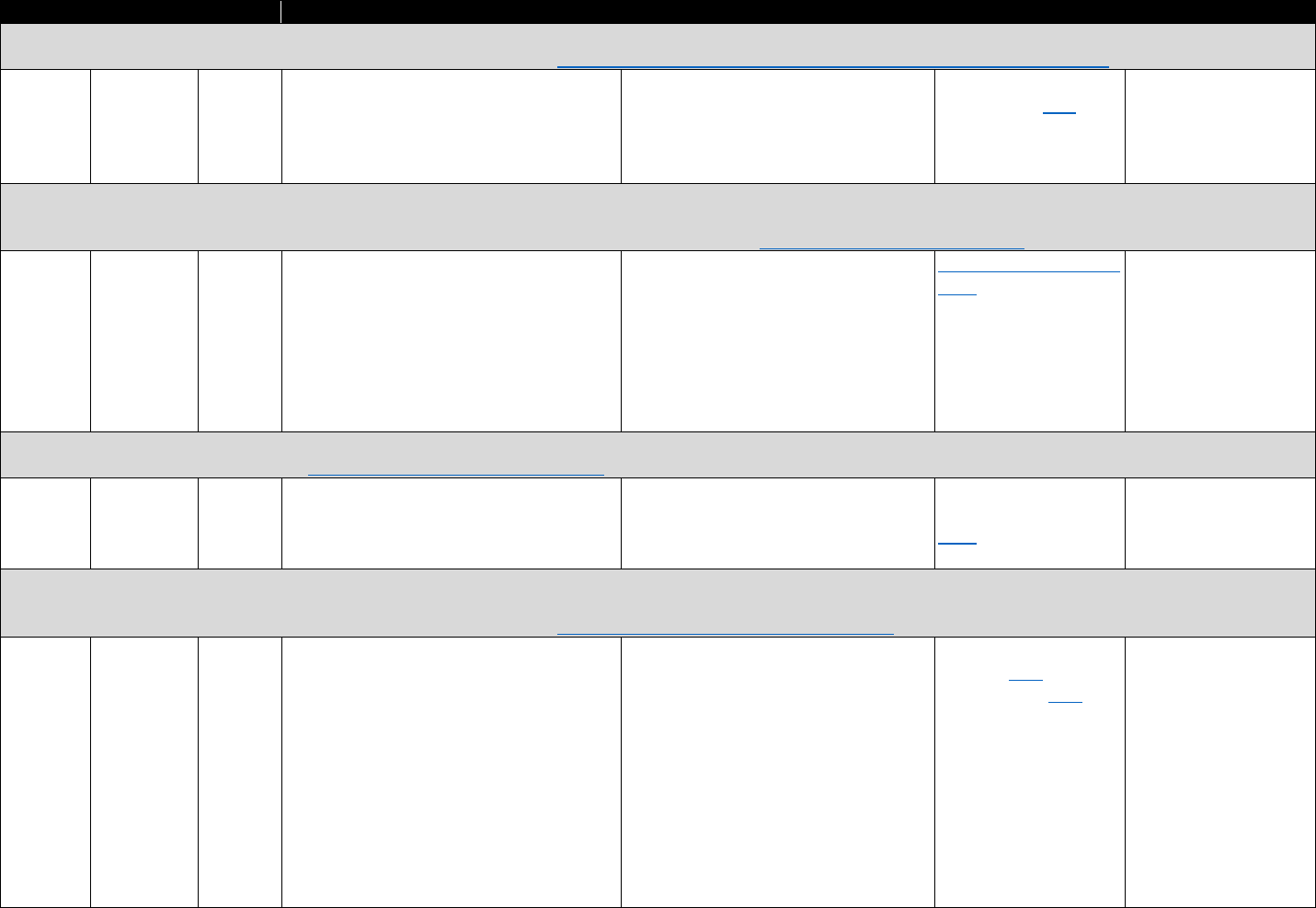
Agency Coverage Timing Measures Costs and Restrictions Available Analytics Linking Capability
Arrestee Drug Abuse Monitoring System (ADAM): Urinalysis results and self-reported drug use and drug use–treatment history collected from adult male arrestees shortly
after their arrests; prevalence estimates are annualized to analyze trends. https://www.nij.gov/topics/drugs/markets/adam/pages/welcome.aspx
National National Annual, Urine screen results, self-reported drug use, No costs stated, some data available only None identified. See No individual-level
Institute of (subset of 1998– self-reported lifetime treatment history, to users at Inter-university Consortium recent report here. linkages identified
Justice jurisdictions 2003 and some information on illicit drug markets for Political and Social Research (ICPSR)
(federal) varies by
year)
2007–
2013
member institutions or upon signing a
Restricted Data Use Agreement
Medical Expenditure Panel Survey (MEPS): A set of large-scale surveys of families and individuals, their medical providers, and employers across the United States. MEPS is
the most-complete source of data on the cost and use of health care (including prescription medications) and health insurance coverage. Data on MEPS participants from
health care providers and facilities are cross-referenced with survey responses from the participants. https://meps.ahrq.gov/mepsweb/
Agency for National Annual Health care visits, use, events, and No costs stated. Researchers and users See online query system MEPS link files to
Healthcare expenditures, names of any prescription with approved projects can access here. National Health
Research medications, and the name and location of restricted data or state/county Interview Survey
and the pharmacy where they obtained the identifiers that have not been publicly person-level public use
Quality prescription. Data on pharmacy-filled released for reasons of confidentiality at data files
(AHRQ) prescription include type, dosage, and the AHRQ Data Center in Rockville,
(federal) payment Maryland, or through the U.S. Census
Research Data Center (RDC) network.
Monitoring the Future Survey (MTF): Nationally representative survey of self-reported drug use by 8th, 10th, and 12th graders. Longitudinal data collection (designed to be
nationally and not state representative). http://www.monitoringthefuture.org/
University National Annual Opioid misuse rates. Contains specific No costs stated. Geographic identifiers None identified. See No individual-level
of questions for OxyContin and Vicodin are not public access recent figures provided linkages identified. Has
Michigan here. been linked with other
(private) state-level information
National Ambulatory Medical Care Survey (NAMCS): Information about the provision and use of ambulatory medical care services based on a sample of visits to non–federally
employed office-based physicians primarily engaged in direct patient care and, starting in 2006, a separate sample of visits to community health centers. Estimates generally
only representative at national or Census region levels (depends on year). https://www.cdc.gov/nchs/ahcd/index.htm
CDC National Annual Utilization of physician, hospital outpatient, No costs stated. Some restricted items Online query system No individual-level
(federal) and emergency department services; the
conditions most often treated; and the
diagnostic and therapeutic services
rendered, including medications prescribed
can only be accessed through National
Center for Health Statistics (NCHS) RDC
available here; other
research tools here
linkages identified
75
Table A.1. National Survey Data
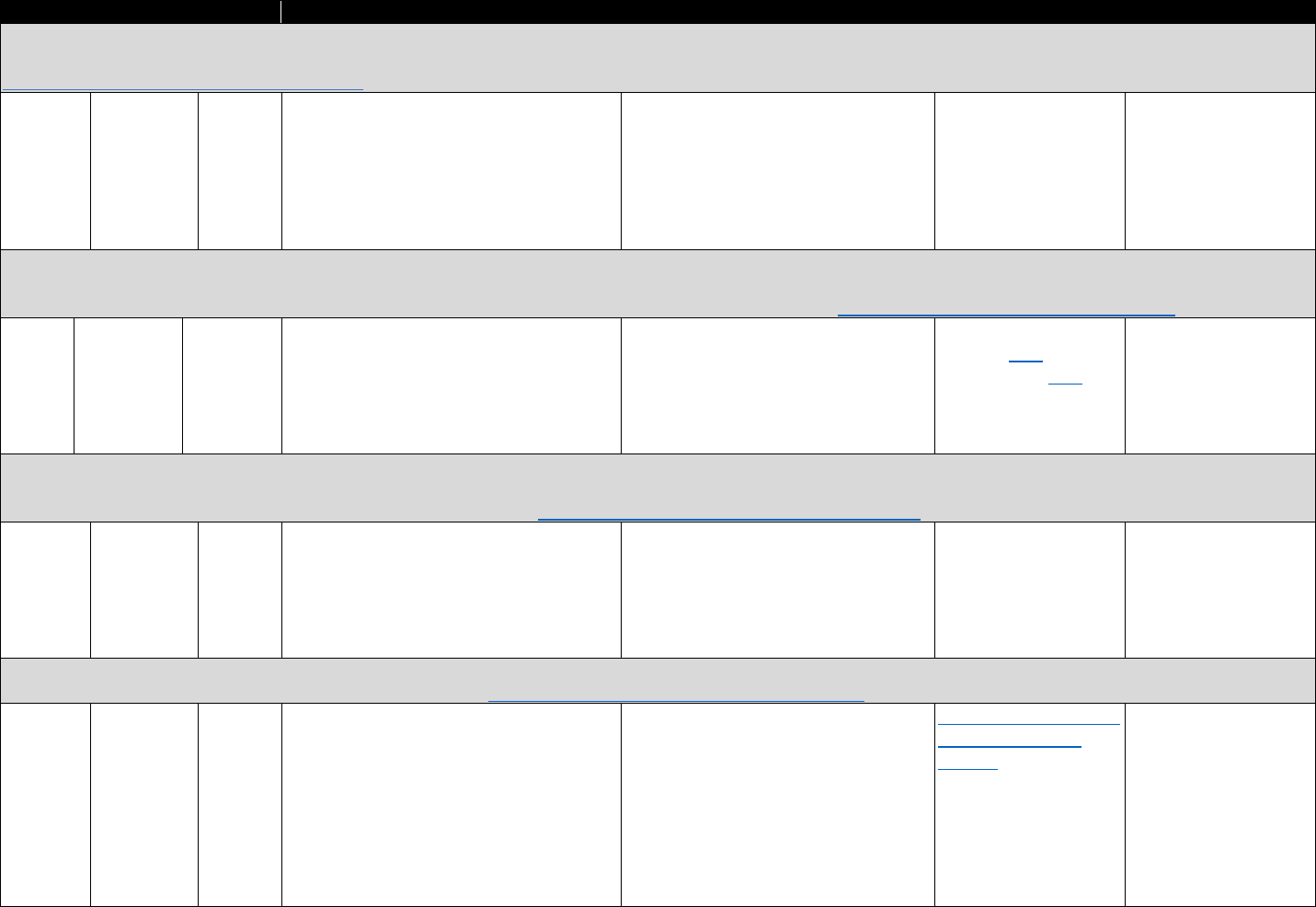
Agency Coverage Timing Measures Costs and Restrictions Available Analytics Linking Capability
National Epidemiologic Survey on Alcohol and Related Conditions (NESARC): This is an epidemiological survey conducted to provide information on mental health, substance,
and psychiatric disorders. Wave I and Wave II were a longitudinal panel. Wave III is a new sample. Sampling is designed to be nationally representative.
https://www.niaaa.nih.gov/research/nesarc-iii
National
Institute
on Alcohol
Abuse and
Alcoholis
(NIAAA)
(federal)
National 2001–
2002,
2004–
2005,
2012–
2013
Nonmedical prescription opioid use and
opioid disorder; mood and anxiety
disorders; other substance use, alcohol
disorder, and other drug use disorder
No costs stated. Only provided to
investigators who agree in advance to
adhere to established policies for
distribution
None identified Access to geocode
identifiers may permit
linkage at state level
National Hospital Ambulatory Medical Care Survey (NHAMCS): Information on the utilization and provision of ambulatory care services in hospital emergency and outpatient
departments and ambulatory surgery locations based on a national sample of visits to the emergency departments, outpatient departments, and ambulatory surgery locations
of noninstitutional general and short-stay hospitals. Estimates only representative at national or Census region. https://www.cdc.gov/nchs/ahcd/index.htm
CDC
(federal)
National Annual Receipt of opioid prescription; visits
specifically for chronic pain condition;
utilization and provision of ambulatory care
services in hospital emergency department,
outpatient departments, and ambulatory
surgery locations
No costs stated. Some restricted items
can only be accessed through NCHS RDC
Online query system
available here; other
research tools here
No individual-level
linkages identified
National Hospital Care Survey (NHCS): NHCS integrates inpatient data formerly collected by the National Hospital Discharge Survey, emergency department and outpatient
department data collected by NHAMCS, and substance-involved visit data previously collected by the Drug-Abuse Warning Network (DAWN). The integration of these three
surveys allows examination of care provided across treatment settings. https://www.cdc.gov/nchs/nhcs/about_nhcs.htm
CDC
(federal)
National,
participating
hospitals
Annual Emergency department
visits for substance abuse and/or resulting
from substance misuse or abuse, adverse
reactions to medications taken as prescribed
or directed, accidental ingestion of drugs,
and drug-related suicide attempts
No costs stated, but fees may apply for
use of the RDC. Access to the data is
allowed through a proposal submission
process and is accessed through the
NCHS RDCs.
Analytics for some
components of the
NHCS available through
ICPSR
Can link with the NDI,
MedPAR, and Medicaid
Statistical Information
System data sets
National Health Interview Survey (NHIS): Data on a broad range of health topics (medical conditions, health insurance, doctor’s office visits, physical activity, and other health
behaviors) are collected through personal household interviews. https://www.cdc.gov/nchs/nhis/about_nhis.htm
CDC
(federal)
National Annual Medical conditions, health insurance,
doctor’s office visits, physical activity, and
other health behaviors
No costs stated. Some variables are
considered restricted data (including
some linkages and geocoded variables)
Online analysis provided
through ICPSR with
account
Can be linked to MEPS,
NDI, Medi-care
enrollment and claims
data, and Social Security
benefit history data
76
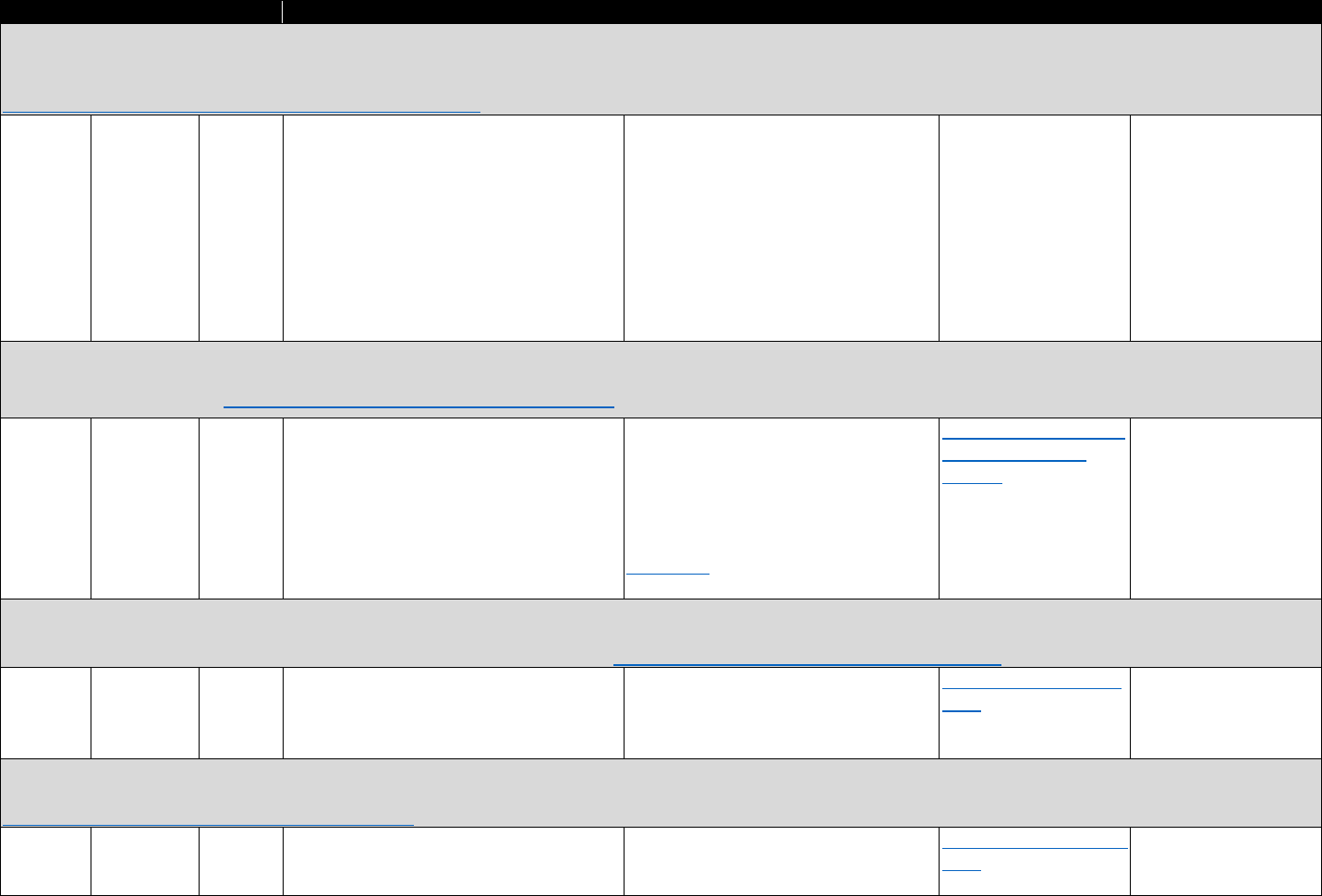
Agency Coverage Timing Measures Costs and Restrictions Available Analytics Linking Capability
National HIV Behavioral Surveillance System (NHBSS): NHBSS collects data relating to behavioral risk factors for human immunodeficiency virus (HIV) (e.g. sexual behaviors,
drug use), HIV testing behaviors, the receipt of prevention services, and use of prevention strategies (e.g. condoms, PrEP). In addition to these interview data, all NHBSS
participants are offered an HIV test. Findings from NHBSS are published in annual reports and other scientific publications.
https://www.cdc.gov/hiv/statistics/systems/nhbs/index.html
CDC National (22 Annual; HIV behavioral risk factors (e.g., sexual No costs stated. Not publicly available; None identified None identified
(federal) city “subject
areas”)
populatio
n cycle
rotation
behaviors, drug use), HIV testing behaviors,
injection drug use, receipt of prevention
services, use of prevention strategies (e.g.
condoms, PrEP)
as a component of HIV/acquired
immunodeficiency syndrome (AIDS)
surveillance, NHBSS data are protected
by the Assurance of Confidentiality
(Section 308[d] of the Public Health
Service Act, 42 U.S.C. 242 m[d]), which
prohibits the disclosure of any
information that could be used to
directly or indirectly identify individuals.
National Survey on Drug Use and Health (NSDUH): Self-reported information on drug use and abuse or dependence, mental health, and substance use disorder treatment
among respondents ages 12 and older. Results available at the national level and for some metropolitan statistical areas and sub-state areas. Designed to be representative at
the national and state levels. https://nsduhweb.rti.org/respweb/homepage.cfm
Substance National Annual Lifetime nonmedical opioid, heroin use; No costs stated. Geographic identifiers Online analysis provided Merged at the state
Abuse and first-time nonmedical opioid use, heroin are restricted access. Restricted access through ICPSR with level with other data
Mental initiates; past-year, past-month heroin, data elements must be applied for and account sets
Health nonmedical opioid, and opioid use by approved, with access to data provided
Administra therapeutic drug class; treatment for opioid through the Substance Abuse and
tion use disorder; self-reported unmet treatment Mental Health Data Archive (SAMHDA)
(SAMHSA) need data portal.
(federal)
National Survey of Substance Abuse Treatment Services Data (N-SSATS): N-SSATS is an annual survey of participating substance use treatment facilities to collect information
on location, characteristics, services offered, and utilization. Information from N-SSATS is used to compile and update the National Directory of Drug and Alcohol Abuse
Treatment Programs and the online Substance Abuse Treatment Facility Locator. https://www.dasis.samhsa.gov/dasis2/nssats.htm
SAMHSA National Annual Type of care provided, including detox and No costs stated. Publicly available State profiles accessible Merged at the county or
(federal) opioid treatment programs, substance
abuse problem treated, types of services
offered, facility funding and capacity
here. state level with other
data sets
Treatment Episodes Dataset (TEDS): Admissions to publicly funded treatment programs and opioid substitution programs by primary, secondary, and tertiary drug, route of
administration, and demographics. Data are available at the national and state levels. Data are submitted from state and local treatment agencies.
SAMHSA
(federal)
National Annual Admissions to treatment facilities (by type;
source of referral) for opioid analgesics or
heroin
No costs stated. Publicly available Data are available online
here.
Linked at the state level
with other information
77
https://wwwdasis.samhsa.gov/webt/information.htm
Agency Coverage Timing Measures Costs and Restrictions Available Analytics Linking Capability
Medicaid State Drug Utilization Data: Drug utilization for sStates are available for covered outpatient drugs paid for by sState Medicaid agencies since the start of the
Medicaid Drug Rebate Program. States are required to report numbers of prescriptions for Medicaid-covered outpatient drugs as well as Medicaid expenditures on the drugs
through Medicaid fee-for-serviceFFS and managed care. https://www.medicaid.gov/medicaid/prescription-drugs/state-drug-utilization-data/index.html
Centers for National Quarterly Drug name, National Drug Code (can Medicaid open data publicly available View data by state Linked at the state level
Medicare and identify specific opioid analgesics), here. Because of privacy restrictions, all online or access CMS with other data sets
Medicaid number of prescriptions, and dollars direct identifiers are removed in the drug spending
Services (CMS) reimbursed public data and aggregate data fewer dashboard here.
(federal) than 11 counts are suppressed.
Medicare Data Files: The Master Beneficiary Summary File includes several segments including enrollment information, chronic conditions data (e.g., mental health, substance
use conditions), service utilization, Medicare payment amounts, and place of residence at the zip-code level. Other notable databases include the Medicare Carrier File (final
action fee-for-service claims submitted on a CMS-1500 claim form); Medicare Outpatient Standard Analytic File (claims and treatment codes); Medicare Provider Analysis and
Review files (hospital inpatient services), and Part D Prescription Drug Event data (contains prescription drug cost and payment data). https://www.resdac.org/cms
-
data/search?f%5B0%5D=im_field_data_file_category%3A46
Research National Annual or Notably, Medicare enrollment, mental May include costs. Varying privacy levels See statistics, trends, Linked at the state,
Data semiannual health and substance use conditions, service for CMS files; requires data use and reports here. county, or zip-code level
Assistance utilization and Medicare payment amounts, agreements to various data sets.
Center death information (only through 2008), and Linked at the person
(ResDAC), prescription drug information level with other
CMS Medicare files; Veterans
(federal) Health Administration
(VHA) data; or Medicaid
claims for Medicare-
Medicaid enrollees
Medicare Part D Prescription Drug Event Data: Every time a beneficiary fills a prescription under Medicare Part D, a prescription drug plan sponsor must submit a summary
record called the prescription drug event (PDE) data to CMS. The PDE record contains prescription drug cost and payment data that enables CMS to make payments to plans
and otherwise administer the Part D benefit. https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovGenIn/PartDData.html
CMS (federal) National Annual Prescription drug costs, payment data,
identifiers, coverage information, and
prescription information
Includes request fee; must be
requested; certain data elements may
be encrypted and/or unavailable
depending on the particular requestor
entity and the demonstrated need for
an element
Medicare Part D Opioid
Mapping Tool
Linked at the state,
county, or zip-code level
to various data sets.
Linked at the person-
level with other
Medicare files; VHA
data; or Medicaid claims
for Medicare-Medicaid
enrollees
78
Table A.2. Claims and Electronic Health Records Secondary Data Sources
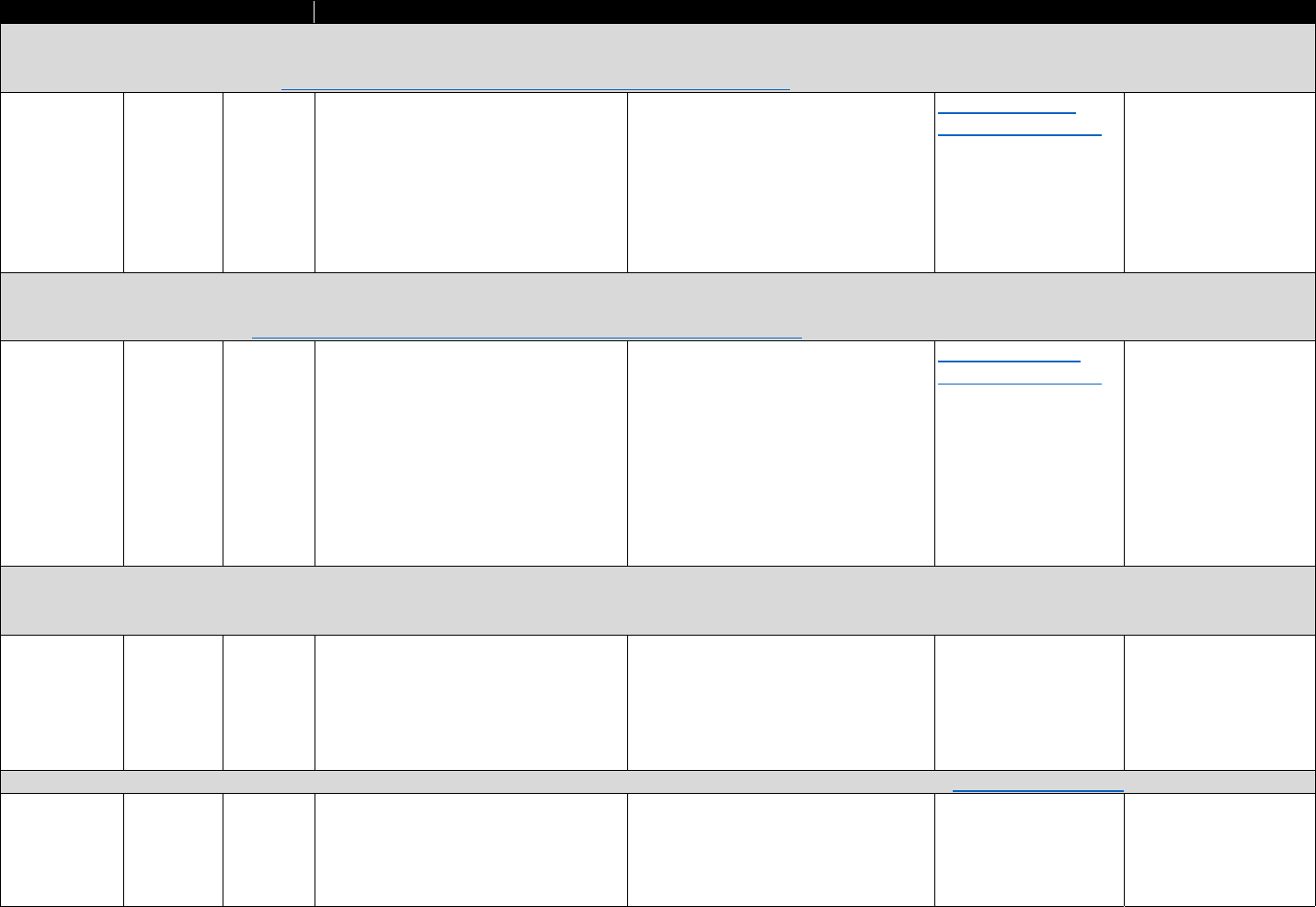
Agency Coverage Timing Measures Costs and Restrictions Available Analytics Linking Capability
Veterans’ Health Administration (VHA) Corporate Data Warehouse (CDW): The CDW and four Regional Data Warehouses (RDW 1–4) were built by the U.S. Department of
Veterans Affairs (VA) Office of Information and Technology to provide a high-performance business intelligence infrastructure through standardization, consolidation, and
streamlining of clinical data systems. https://www.hsrd.research.va.gov/for_researchers/vinci/cdw.cfm
VA (federal) National Near real-
time
Patient-level data on prescriptions and
health care utilization
This data set is not for public access or
use. Research requests must go through
the Data Access Request Tracker
application. With approval, data access
to CDW can be obtained from CDW
through approved SQL tables delivered
to a research project or accessed
through SAS Proc SQL.
Maps of VA opioid
prescribing data here.
Links data across
multiple VHA data
source system
VHA National Patient Care Database: The National Patient Care Database (NPCD), which is housed at the Austin Information Technology Center, is part of the National
Medical Information Systems (NMIS). The NPCD collects integrated patient care data from all Veterans Health Information Systems and Technology Architecture (VistA)
information technology systems. https://www.data.va.gov/dataset/national-patient-care-database-npcd
VA (federal) National Updated
daily
Clinical data resulting from ambulatory
care patient encounters; primary care
patient to provider assignments and
provider utilization data
This data set is not for public access or
use. Research requests must go through
the Data Access Request Tracker
application. With approval, data access
to CDW can be obtained from CDW
through approved SQL tables delivered
to research project or accessed through
SAS Proc SQL.
Maps of VA opioid-
prescribing data here.
Can be linked with other
VHA patient-level data
systems and across
years to generate
episodes of care for
individuals; can be
linked with mortality
data by Social Security
Number
Clinical Data Base/Resource Manager of Vizient Inc.: The Vizient Clinical Data Base and Resource Manager™ (formerly University Helathsystem Consortium) is an
administrative, clinical, and financial database providing clinical, discharge, procedure, and outcome data for hospital encounters from a consortium of hospitals and academic
health centers.
Vizient Inc.
(private)
National
(across
network)
Not stated Patient outcome data including
mortality, length of stay, complication
rates, and readmission rates—can
categorize by opioid use (does not
appear to collect dose information)
Costs not stated; may need to be a
Vizient member to access data
None identified May be linked at the zip-
code tabulation area or
more-aggregate level;
supports linkage with
American Hospital
Association survey data
EHRs from Group Health Cooperative (GHC, now Kaiser Permanente): Information from EHRs of patients in the GHC network. https://www.ghc.org/
Kaiser (private) Washington
state
Near–real
time
Prescribing of opioids, past prescribing,
and reason for admission (opioids
related)
Costs not stated; unclear if data are
available for online analysis
None identified
79
https://www.vizientinc.com/Our-solutions/Clinical Solutions/Clinical-Data Base
Has been linked at the
individual level to
mortality data and
traffic accident data
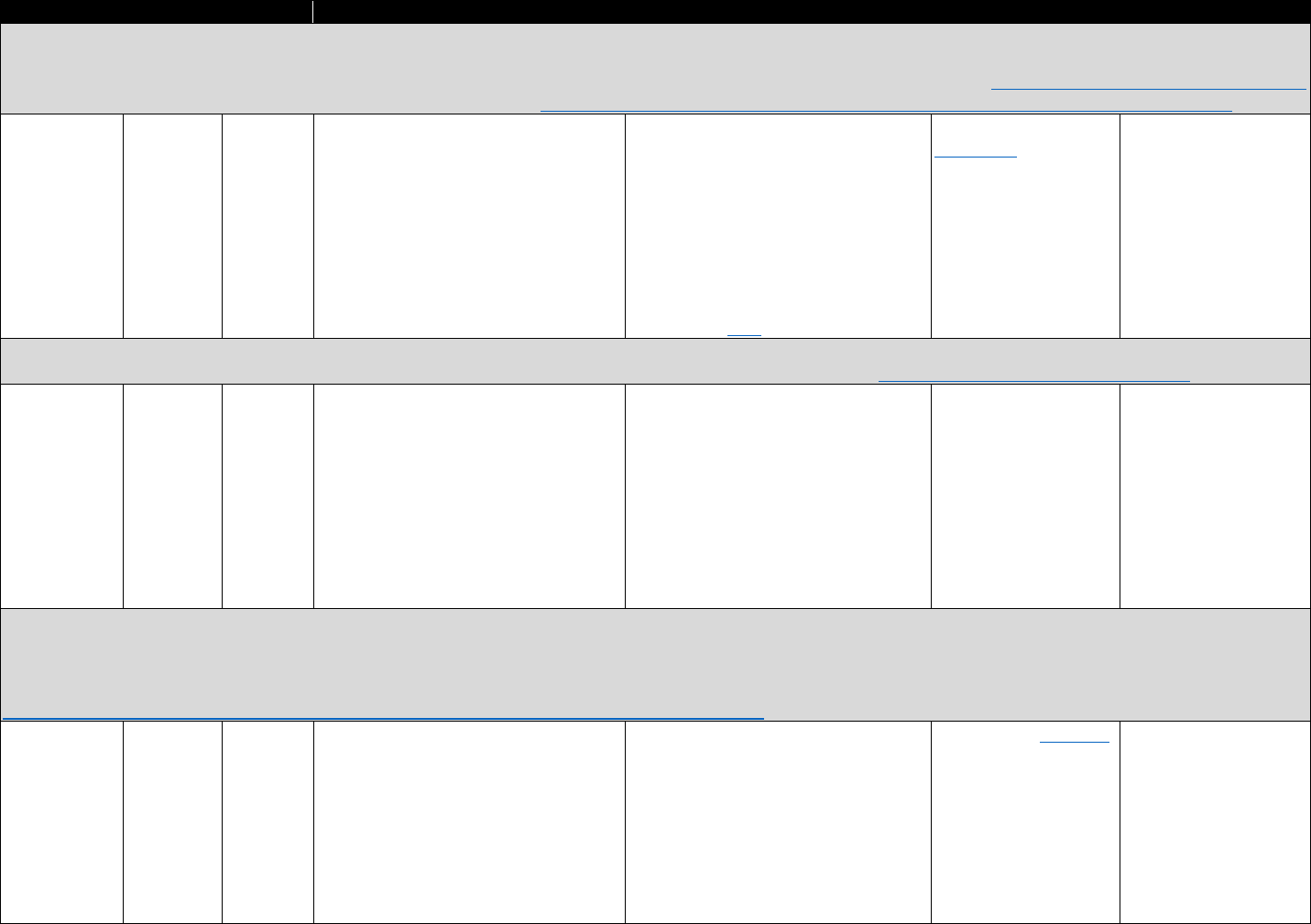
Agency Coverage Timing Measures Costs and Restrictions Available Analytics Linking Capability
Stanford Translational Research Integrated Database: The Stanford Translational Research Integrated Database has three integrated components: a clinical data warehouse,
based on the HL7 Reference Information Model, containing clinical information on over 1.3 million pediatric and adult patients since 1995; an application development
framework for building research data management applications on the data platform; and a biospecimen data management system. http://med.stanford.edu/researchit.html
Replaced by the STAnford medicine Research data Repository in 2017: http://med.stanford.edu/researchit/infrastructure/clinical-data-warehouse/starr-faq.html
Stanford
University
(private)
Stanford
University
Medical
Center
Real time Prescribing information; has been used
to identify patient research cohorts by
condition
Costs not stated. Identified clinical data
in the CDW is only released to
Institutional Review Board (IRB)–
approved research studies that have
received the appropriate IRB approval.
De-identified data are made available
for Stanford research projects that
qualify as a nonhuman subject research
study. Answers to data-
access questions
are available here.
Online access to the
cohort tool only through
connection to Stanford
network or virtual
private network
The Stanford
Translational Research
Integrated Database
exists in part as a tool
for data linkages,
although no linkages
specific to opioids
identified.
HealthCore Integrated Research Database: Integrated database of commercially insured population. Contains medical and pharmacy administrative claims data plus health
plan eligibility information on enrollees in large commercial insurance plans (Blue Cross/Blue Shield) across 14 states. https://www.healthcore.com/database/
HealthCore
(private)
National
(subset of
states)
Not stated Insurance holder demographics, claims
data relevant for opioid use, including
emergency department visits and
adverse drug events; prescription
information
Costs not stated. Data primarily
available only through consultants.
HealthCore does not sell data to third
parties for their independent use or
otherwise.
None identified Linked with hospital,
local, and federal data
MarketScan commercial claims database: The MarketScan commercial claims and encounters database consists of employer- and health plan–sourced data containing
medical and drug data for several million individuals annually. Health care for these individuals is provided under a variety of fee-for-service, fully capitated, and partially
capitated health plans, including preferred and exclusive provider organizations (PPOs and EPOs), point-of-service plans, indemnity plans, health maintenance organizations
(HMOs), and consumer-directed health plans. Medical claims are linked to outpatient prescription drug claims and person-level enrollment information.
https://truvenhealth.com/your-healthcare-focus/analytic-research/marketscan-research-databases
Truven Health
Analytics
(private)
National Quarterly Prescribing trends, rates of opioid
prescribing
Costs vary. Customized data sets and
licensing agreements available.
Accessing the data requires data
management software. DataProbe® and
MarketScan Online Tools (e.g., Sample
Select, Sample Select Prevalence,
Inpatient View, Outpatient View,
Disease Profiler, Treatment Pathways)
can facilitate access.
Online access available,
but must be purchased
Can be linked with other
MarketScan databases
80

Agency Coverage Timing Measures Costs and Restrictions Available Analytics Linking Capability
MarketScan Multi-State Medicaid Database: The MarketScan Medicaid database contains standardized, fully integrated, enrollee-level de-identified claims across inpatient,
outpatient, and prescription drug services for both fee-for-services and capitation plans. Data on eligibility (by month) and service and provider type are also included. In
addition to standard demographic variables such as age and gender, the database includes variables of particular importance for investigating Medicaid populations, such as
aid category (blind/disabled, Medicare eligible) and race. Data are collected from employers, health plans, or state Medicaid agencies. https://truvenhealth.com/your
-
healthcare-focus/analytic-research/marketscan-research-databases
Truven Health
Analytics
(private)
Multistate
(12 states in
2010)
Semiannua
lly
Pharmaceutical claims for filled
prescriptions, outpatient service claims
records, inpatient admissions records
Costs vary. Customized data sets and
licensing agreements available.
Accessing the data requires data
management software. DataProbe® and
MarketScan Online Tools (e.g., Sample
Select, Sample Select Prevalence,
Inpatient View, Outpatient View,
Disease Profiler, Treatment Pathways)
can facilitate access.
Online access available,
but must be purchased
Can be linked with other
MarketScan databases
Optum database: Large database of eligibility-controlled claims information (commercial and Medicare members of affiliated plans, and commercial members of Optum
Employer customers’ and Optum Payer customers’ health plans). Comprises complete inpatient, outpatient, and pharmacy claims.
Optum
(private)
National Not stated Opioid episode duration and dosage;
opioid overdose; enrollment, utilization,
all available clinical data in EMR/EHR
Costs and access restrictions not stated. None identified State (and possibly
county) identifiers
support linkage at
aggregate level
Symphony Health Solutions’ Integrated Dataverse: Comprehensive source providing insight to all the factors that drive pharmaceutical brand success—medical, hospital and
prescription claims, and point-of-sale prescription data, nonretail invoice data, and demographic data; designed more for market research than policy research. It contains
pharmacy retail transactions from more than 80 percent of pharmacies nationwide, including high-volume national chain pharmacies, resulting in information on
approximately 90 percent of prescriptions filled at retail pharmacies in the United States. Missing pharmacies are generally independent or part of small chains. Symphony
obtains pharmacy data directly from prescription drug claim processors and payers, using the same data that get verified against standard reporting information to the U.S.
government. https://symphonyhealth.com/product/idv/
Symphony
Health (private)
National Not stated Medical, hospital and prescription claims
related to opioid prescribing and/or
overdose, point-of-sale prescription
data, nonretail invoice data, and
demographic data
Costs vary by request None identified Can be merged with
other state- or county-
level information
IQVIA (formerly IMS) National Disease and Therapeutic Index (NDTI): The NDTI is a monthly audit of office-based physicians that provides information about patterns and
treatment of disease in the continental United States. For each patient seen during a consecutive two-day period each calendar quarter, participating physicians complete an
encounter form that includes information about diagnoses and drug therapies. Each record of a drug therapy within the NDTI is linked to a specific six-digit taxonomic code
capturing diagnostic information similar to the ICD-9. http://www.imshealth.com/en and https://www.iqvia.com
IQVIA (private) National Monthly;
quarterly
analysis
suggested
Diagnosis codes; underlying and
concomitant conditions; prescription
information; drug appearance or drug
use; patient and physician characteristics
Costs vary depending on request Available (with
payment) via the
customer portal
Can be merged with
other state- or county-
level information
81
https://www.optum.com/solutions/data
analytics/data/real-world-data-analytics-a-cpl/claims-data.html
-
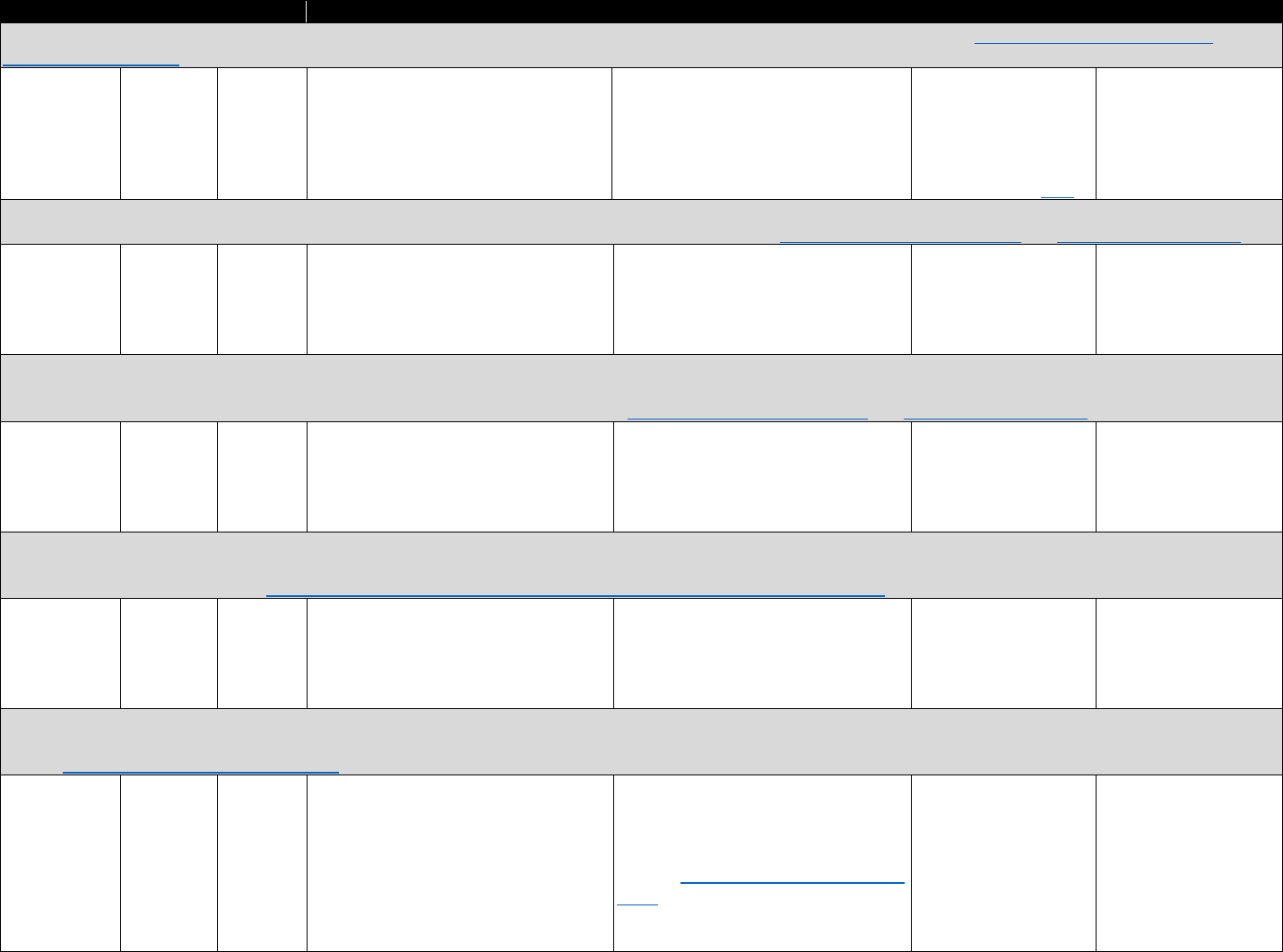
Agency Coverage Timing Measures Costs and Restrictions Available Analytics Linking Capability
IQVIA (formerly IMS) National Prescription Audit: Measures retail dispensing of prescriptions to consumers via formal prescriptions. http://www.imshealth.com/en and
https://www.iqvia.com
IQVIA (private) National Monthly Prescriptions (by National Drug Code),
channel (i.e., where prescription filled),
prescriber specialty
Costs vary depending on request.
Geographic identifiers not available
below three zip-code levels
Available (with
payment) via the
customer portal. The
CDC has online graphs
of aggregate data by
state and county here
Can be merged with
other state- or county-
level information
IQVIA (formerly IMS) National Sales Perspectives: Measures sales volume of dollars and units of pharmaceutical products purchased by retail and nonretail providers. Data
collected from a large sample of manufacturers, wholesalers, outlets, and projected to national estimates. http://www.imshealth.com/en/ and https://www.iqvia.com/
IQVIA (private) National
(projected)
Monthly Prescription sales volume (by product
type), number of units sold
Costs vary depending on request. Flat
files can be delivered through secure
File Transfer Protocol platform
Available (with
payment) via the
customer portal
Projected data intended
for national analyses;
however, state or
county linkages may be
possible
IQVIA (formerly IMS) PayerTrak: PayerTrak is a web-based approach to trends in prescription drug utilization by payer. PayerTrak provides access to payer prescription volume
in all markets and all payers within the retail channel. With the PayerTrak tool, subscribers can quickly assess market share and copay for desired prescription products or
prescription markets in an easy-to-use tool. Data are projected to national estimates. http://www.imshealth.com/en/ and https://www.iqvia.com/
IQVIA (private) National
(projected)
Monthly Total prescriptions (by product), pay
type, state, copay
Costs vary depending on request Available (with
payment) via the
customer portal
Projected data intended
for national analyses;
however, state or
county linkages may be
possible
Massachusetts Medicaid Claims and Enrollment Data (MassHealth): Massachusetts state insurance data on claims. MassHealth claims and encounter data provided a
comprehensive history of health care utilization and expenditures, as well as associated diagnoses, in both general medical and behavioral health services sector across a
broad range of health care settings. http://www.mass.gov/eohhs/provider/insurance/masshealth/claims/claims-data/
Massachusetts
Health and
Human
Services (state)
Single state Annual
(may be
possible at
other
levels)
Treatment for addictions, diagnosis of
opioid dependence, expenditures on
treatment, mortality (in the eligibility
file)
Not stated None identified Has been merged with
other state data sets at
the individual level
Massachusetts All-Payer Claims Database (MA APCD): The MA APCD is the most comprehensive source of health claims data from public and private payers in Massachusetts.
With information on the vast majority of Massachusetts residents, the MA APCD promotes transparency and affords a deep understanding of the Massachusetts health care
system. http://www.chiamass.gov/ma-apcd/
Massachusetts
Center for
Health
Information
and Analysis
(state)
Single state Annual
(may be
possible at
other
levels)
Health and pharmacy insurance claims
related to opioids or other prescription
medication, infant diagnosis codes for
neonatal abstinence syndrome,
demographics
Fees may apply. Data must be
requested and approved. See links to
"Steps to Request the Data" for
government and non-government
entities: http://www.chiamass.gov/ma
-
apcd/
None identified Forms the spine of the
Chapter 55 data set,
linked to mortality,
prescription drug
monitoring program
(PDMP), criminal justice,
treatment, and other
data sets
82
Agency Coverage Timing Measures Costs and Restrictions Available Analytics Linking Capability
Vermont Health Care Uniform Reporting and Evaluation System (VHCURES): Vermont’s APCD, a comprehensive, longitudinal, multipayer data set that regularly collects
medical and pharmacy claims data and eligibility data from both private and public payers. http://gmcboard.vermont.gov/hit/vhcures
Vermont Green
Mountain Care
Board (state)
Single state Annual
(may be
possible at
other
levels)
Medical expenditures, costs of treatment
for opioid use disorders
Costs apply. Through data use
agreements, de-identified VHCURES
data is being utilized by state agencies,
state contractors, and academic
researchers to support analysis of
health care access, spending, utilization,
and quality.
None identified None identified
83
Agency Coverage Timing Measures Costs and restrictions Available analytics Linking capability
CDC WONDER Multiple Cause of Death Data: The Multiple Cause of Death data available on CDC WONDER provide county-, state-, and national-level mortality and
population data. Data are based on death certificates for U.S. residents. Each death certificate contains a single underlying cause of death, up to 20 additional multiple
causes, and demographic data. https://wonder.cdc.gov/mcd.html
CDC National Released Number of deaths, crude death rates, age- No costs and publicly available. Online data portal here Merged with other
(federal) annually
(but can
obtain
monthly
aggregate)
adjusted death rates (can be analyzed by
drug and alcohol related causes of death,
injury intent and injury mechanism
categories)
Subnational data representing zero to
nine deaths are suppressed
state- or county-level
information
National Death Index (NDI): The NCHS established the NDI as a resource to aid epidemiologists and other health and medical investigators with their mortality-ascertainment
activities. https://www.cdc.gov/nchs/ndi/index.htm
CDC National Annual Study participant death, dates of death, and Fee per study subject with fee schedule None identified Can be linked at the
(federal) the corresponding death certificate numbers.
NDI Plus provides cause of death
here. NDI service is available to
investigators solely for statistical
purposes in medical and health
research. The service is not accessible
to organizations or the general public
for legal, administrative, or genealogy
purposes.
individual level to the
NHIS; National Health
and Nutrition
Examination Survey;
longitudinal study of
aging; and VA health
care data; has been
linked with a variety of
state-specific health
data sets
National Vital Statistics System (NVSS) Multiple-Cause-of-Death files: Mortality data from NVSS are a fundamental source of demographic, geographic, and cause-of-death
information. Comparable for small geographic areas and available for a long time period in the United States. The data are used to present the characteristics of those dying
in the United States to determine life expectancy and to compare mortality trends. https://www.cdc.gov/nchs/nvss/mortality_methods.htm and
http://www.nber.org/data/vital-statistics-mortality-data-multiple-cause-of-death.html
CDC
(federal)
National Annual Mortality with information on drugs involved
in death
No costs. Microdata files must be
requested and approved before being
provided on CD or DVD.
NVSS is the underlying
data for CDC WONDER
Merged at the county-
level with other data
sets
Fatal Accident Reporting System (FARS): Data derived from a census of fatal traffic crashes within the 50 states, the District of Columbia, and Puerto Rico primarily from the
police accident report in those states, but also from death certificates, state coroners and medical examiners, state driver and vehicle registration records, and emergency
medical services records. https://www.nhtsa.gov/research-data/fatality-analysis-reporting-system-fars
National National Annual 143 different coded data elements (as of No costs and publicly available See online query system Geocode identifiers
Highway 2013) that characterize the fatal crash, here. support linkage at the
Traffic including toxicology reports city, county, and state
Safety levels.
Administ
ration
(federal)
84
Table A.3. Mortality Records
Agency Coverage Timing Measures Costs and restrictions Available analytics Linking capability
Examples of State Death Certificate Data Provided Below
Florida Department of Health mortality data: http://www.floridahealth.gov/statistics-and-data/
Death certificate data for the state of Florida containing information on cause of death
Data access: application form and information provided here
Prior studies using this data: Kennedy-Hendricks et al. (2016)
Past linkages: Merged at the state level with Florida PDMP information
North Carolina State Center for Health Statistics: http://www.schs.state.nc.us/aboutus.htm
Death certificate data for the state of North Carolina containing information on cause of death
Data access: Requests requiring extensive analysis or computer programming may be subject to a charge and completed as available staff time permits.
Prior studies using these data: Albert et al. (2011); Hirsch et al. (2014); Dasgupta et al. (2016); Kennedy-Hendricks et al. (2016)
Past linkages: Linked at the individual level, matching decedent names to controlled substance–prescription histories through PDMP data
North Carolina Office of the Chief Medical Examiner: http://www.ocme.dhhs.nc.gov/
Detailed data on all deaths in North Carolina caused by injury or violence, as well as natural deaths that are suspicious, unusual, or unattended by a medical professional;
contains postmortem serum toxicological analyses
Data access: Autopsy, investigation, and toxicology reports are also public records and once finalized, may be obtained from the Office of the Chief Medical Examiner. To
request any of these documents, use the Document Request web form.
Prior studies using these data: Albert et al. (2011); Hirsch et al. (2014); Dasgupta et al. (2016)
Past linkages: Linked at the individual level to state death certificate data and state PDMP data
Massachusetts Registry of Vital Records and Statistics: http://www.mass.gov/eohhs/gov/departments/dph/programs/admin/dmoa/vitals/
Vital records and deaths for Massachusetts
Data access: Information provided here
Prior studies using these data: Walley et al. (2013); The Commonwealth of Massachusetts, Executive Office of Health and Human Services, Department of Public Health
(2016, 2017)
Past linkages: Linked at the individual level to multiple other state databases under Chapter 55 (see The Commonwealth of Massachusetts, Executive Office of Health and
Human Services, Department of Public Health [2016, 2017])
Tennessee Department of Health, Division of Health Statistics, Death Statistical System: https://www.tn.gov/health/health-program-areas/statistics/health-data/
death-statisticshtml
Cause of death statistics for Tennessee (note: Tennessee Department of Health has many public health statistics publicly available)
Data access: Individual-level data not publicly available. Contact department for further information.
Prior studies using this data: Baumblatt et al. (2014)
Past linkages: Linked at the individual level to state PDMP data
85
Agency Coverage Timing Measures Costs and Restrictions Available Analytics Linking Capability
Automation of Reports and Consolidated Orders System (ARCOS): Measure of prescription drug supply based on mandatory reporting for Schedule I and II controlled
substances and selected Schedule III and IV substances from manufacture to sale. Data for each substance reported by quantity (e.g., mg, dosage unit) and three-digit zip
code. https://www.deadiversion.usdoj.gov/arcos/
Drug National Annual Amount of manufactured controlled Costs not stated. Available to all DEA Summary reports Merged with other data
Enforcem substance circulating through legal means, manufacturers and distributers; must publicly available sources at the county or
ent by compound procure data through Freedom of state level
Administr Information Act (FOIA) request; public
ation data are usually released only at the
(DEA) state level, but three-digit zip-level data
(federal) have been used under special agreement
Prescription Behavior Surveillance System: Epidemiological surveillance and evaluation tool based on de-identified longitudinal data from state PDMPs to measure trends in
controlled substance prescribing and dispensing and indicators of medical use and possible nonmedical prescription drug abuse and diversion.
http://www.pdmpassist.org/content/prescription-behavior-surveillance-system
TTAC @ 12 states Quarterly Forty-three prescription behavior Costs not stated. Data-sharing Online access for Compiles PDMP
Brandeis submitting; measures: overall usage within drug classes agreement specifies how Brandeis will authorized federal information across states
(federally more being and for selected individual drugs; daily manage, secure, and protect the PDMP researchers
funded) reviewed to
join
dosage; overlapping prescriptions within
each drug class or across classes;
questionable activity; payment source;
indicators of possible pill mills;
inappropriate prescribing measures; and
pharmacy-based measures of possible
inappropriate dispensing
data; data are maintained securely at
Brandeis, and access by Brandeis
research staff is limited in accordance
with the IRB-approved protocol.
Procedures are in process to provide
access by authorized federal researchers.
Examples of State Prescription Drug–Monitoring Program (PDMP) Data
Maine Prescription Monitoring Program: http://www.maine.gov/dhhs/samhs/osa/data/pmp/index.htm
Maine’s PDMP data, hosted by the Maine Substance Abuse and Mental Health Services
Data access: Agency has demonstrated willingness to provide data sets needed for research to address the problem of opioid misuse and abuse. De-identified data have been
made available to researchers.
Prior studies using this data: Piper et al. (2016), Kreiner et al. (2017)
Past linkages: Linked at the individual level to other prescriber information; merged at the county level with Maine Diversion Alert Program data
Maryland Prescription Drug Monitoring Program: https://bha.health.maryland.gov/pdmp/Pages/Home.aspx
Maryland’s PDMP data, hosted by the Maryland Department of Health and Mental Hygiene, Behavioral Health Administration
Data access: Individuals requesting data must complete training prior to submitting any data requests.
Prior studies using this data: Lin et al. (2016)
Past linkages: Linked at the prescriber level to a different survey on physician attitudes and use of PDMP
86
Table A.4. Prescription Monitoring Secondary Data Sources

Massachusetts Prescription Drug Monitoring Program: http://www.mass.gov/eohhs/gov/departments/dph/programs/hcq/drug-control/pmp/reports-and-data.html
Massachusetts’s PDMP data, hosted by the Massachusetts Department of Public Health
Data access: Data request form available here
Prior studies using this data: Katz et al. (2010), The Commonwealth of Massachusetts, Executive Office of Health and Human Services, Department of Public Health (2016,
2017)
Past linkages: Linked at the individual level to multiple other state databases under Chapter 55 (see The Commonwealth of Massachusetts, Executive Office of Health and
Human Services, Department of Public Health [2016, 2017]); also allows interstate data sharing
Tennessee Controlled Substances Monitoring Program/Database:
Tennessee’s PDMP data, hosted by the Tennessee Department of Health
Data access: The law allows a number of other state and federal officials to register with the database, including certain law enforcement officers, medical examiners, drug
court judges, and others.
Prior studies using this data: Baumblatt et al. (2014)
Past linkages: Linked at the individual level to state death certificate data
Ohio Automated Rx Reporting System: https://www.ohiopmp.gov/
Ohio’s PDMP data, hosted by the State of Ohio Board of Pharmacy
Data access: Not stated
Prior studies using this data: Baehren et al. (2010), Weiner et al. (2017)
Past linkages: Linked at the individual level with patient emergency department data
Kentucky All Schedule Prescription Electronic Reporting System:
Kentucky’s PDMP data, hosted by the Kentucky Cabinet for Health and Family Services
Data access: Not stated
Prior studies using this data: Blondell et al. (2004), Brady et al. (2014), Becker et al. (2017), Slavova et al. (2017)
Past linkages: Merged with zip-, county-, or state-level social and economic variables. The Kentucky Department of Public Health, Cabinet for Health and Family Services, has
established a multisource drug-overdose surveillance system, including the PDMP and various other state data sources (e.g., emergency department discharges, overdose
death and postmortem toxicology, and heroin/fentanyl submissions to Kentucky State Police crime labs).
Florida’s Prescription Drug Monitoring Program: http://www.floridahealth.gov/statistics-and-data/e-forcse/
Florida’s PDMP data, hosted by the Florida Department of Health
Data access: Not stated
Prior studies using this data: Delcher et al. (2015)
Past linkages: Merged with other state-level data sources (e.g., mortality)
87
https://www.tn.gov/health/health-program-areas/health-professional-boards/csmd-board.html
https://chfs.ky.gov/agencies/os/oig/dai/deppb/Pages/kasper.aspx
North Carolina Controlled Substances Reporting System: https://nccsrsph.hidinc.com/nclogappl/bdncpdmqlog/pmqhome
https://www.ncdhhs.gov/divisions/mhddsas/ncdcu/csrs
North Carolina’s PDMP data, hosted by the North Carolina Department of Health and Human Services, Division of Mental Health, Developmental Disabilities, and Substance
Abuse Services
Data access: Permission to query the system must be obtained from system administrators.
Prior studies using this data: Albert et al. (2011), Hirsch et al. (2014), Ringwalt et al. (2015), Dasgupta et al. (2016), Roberts et al. (2016)
Past linkages: Linked at the individual level with Medicaid claims data and mortality data
California’s Controlled Substance Utilization Review and Evaluation System: https://oag.ca.gov/cures
California’s PDMP data, hosted by the California Department of Justice
Data access: For access, researchers must obtain a background check from the California Department of Justice. An SQL server is used within the Department of Justice to de-
identify the database using a record-linkage methodology to permit identification of sequential prescriptions for each patient. Unique computer-generated identifiers are
devised for each provider and pharmacy to remove identifying information at the patient, provider, or pharmacy level.
Prior studies using this data: Wilsey et al. (2011), Gilson et al. (2012), Han et al. (2014)
Past linkages: No individual-level linkages identified.
Oregon’s Prescription Drug Monitoring Program: http://www.orpdmp.com/researchers.html
Oregon’s PDMP data, hosted by the Oregon Health Authority
Data access: The Oregon Health Authority may provide de-identified PDMP data for research purposes. The Oregon Health Authority is accepting research requests.
Prior studies using this data: Hartung et al. (2012); O'Kane et al. (2016); Deyo et al. (2017)
Past linkages: Linked at the patient level to state vital records, hospital discharge registry, and Medicaid administrative pharmacy claims
88
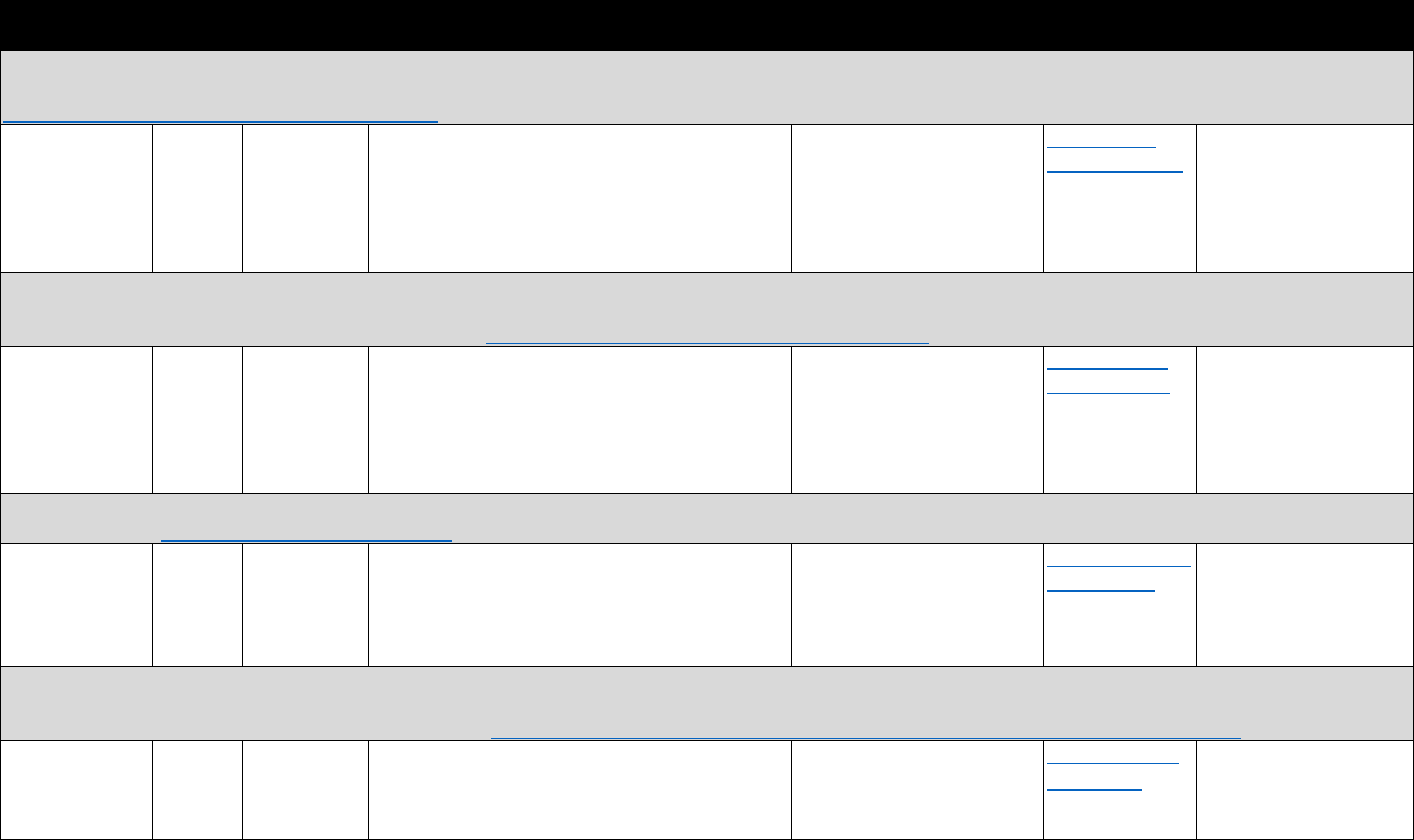
Agency Coverage Timing Measures Costs and Restrictions
Available
Analytics Linking Capability
Area Health Resource Files (AHRF): The AHRF data include county, state, and national-level files in eight broad areas: health care professions, health facilities, population
characteristics, economics, health professions training, hospital utilization, hospital expenditures, and environment. The AHRF data are obtained from more than 50 sources.
https://www.hrsa.gov/about/contact/ehbhelp.aspx
Health Resources National Annual Information on health care cost and utilization, No costs and publicly See tools and Merged county-level
and Services (some demographics, health care facilities and services, available data portal here. contextual factors with
Administration measures are vital events, and other health information based other data on opioid
Data Warehouse available daily, on geographic region outcomes
(federal) monthly, and
quarterly)
Current Population Survey (CPS): Primary source of labor force statistics for the U.S. population. Supplemental questions are added to the basic CPS questions;
supplemental inquiries vary month to month and cover a wide variety of topics such as child support, volunteerism, health insurance coverage, and school enrollment.
Supplements are usually conducted annually or biannually. https://www.census.gov/programs-surveys/cps.html
U.S. Census National Monthly Information on educational status, health No costs and publicly See interactive Merged state- or county-
Bureau and the insurance, work and labor market outcomes, available. Not all counties are data tools here. level contextual factors
U.S. Bureau of income, disability, household characteristics (e.g., included, and data are not with other data on opioid
Labor Statistics household size), demographics (e.g., age, race, available for most sampled outcomes
(federal) gender), labor force participation, and poverty
rates
counties due to
confidentiality laws.
National Alliance Model for State Drug Laws (NAMSDL) policy data: Provides information on current state statutes and policies related to controlled substances and
prescription drugs. http://www.namsdl.org/index.cfm
NAMSDL National Updated Statutes related to naloxone access; pain No costs and publicly See maps of state Merged with state-level
(federally funded) semiannually management, pain clinics, and prescribing
practices; Good Samaritan Laws; PDMPs; doctor
shopping laws; prescription trafficking statutes;
regulation of internet pharmacies
available. Historical data are
not available or readily
downloadable for all policies.
policies here. data on opioid-related
outcomes
National Conference of State Legislatures (NCSL) policy data: NCSL maintains legislative tracking databases about public health issues such as criminal justice, education,
employment policy, immigrant policy, transportation, health care access, and public health. Users can search tracking databases for relevant legislation by year, topic, and
keyword. Users can download state legislation as a PDF file. http://www.ncsl.org/research/health/ncsl-prescription-drug-policy-resources-center.aspx
NCSL
(nongovernment
al organization)
National Annual State legislation related to Medicaid prescription
drug policies; PDMPs; prescribing guidelines;
naloxone; pain clinics
No costs and publicly
available. Historical data are
not available or readily
downloadable for all policies.
Online database
search here
.
Merged with state-level
data on opioid-related
outcomes
89
Table A.5. Contextual and Policy Data Sources
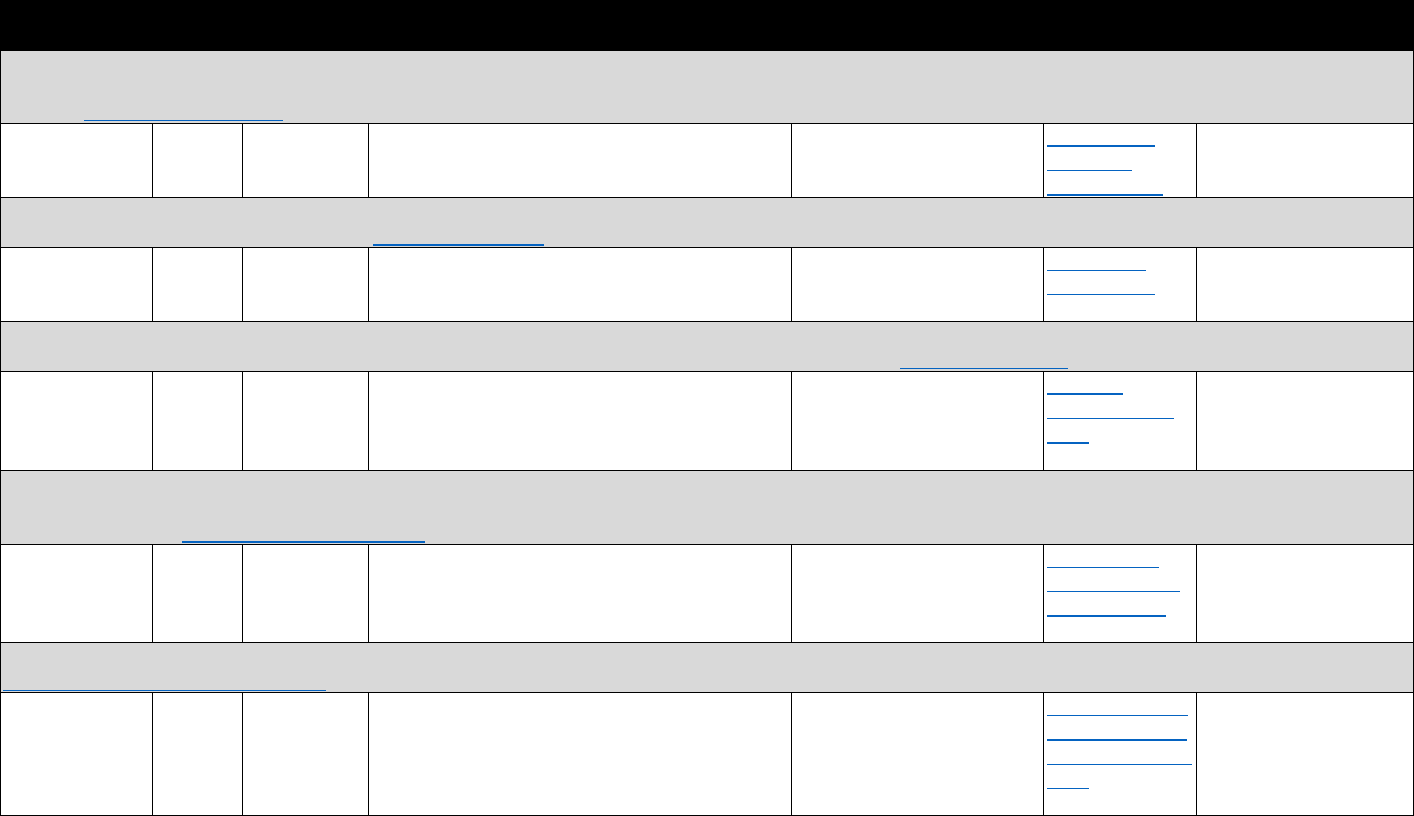
Agency Coverage Timing Measures Costs and Restrictions
Available
Analytics Linking Capability
Prescription Drug Abuse Policy System (PDAPS): Tracks key state laws related to prescription drug abuse. PDAPS provides accurate, detailed information about important
policies designed to promote the safe use of controlled medicines and reduce overdoses. PDAPS users interact with and download legal data through the MonQcle software
platform. http://www.pdaps.org/
Legal Science, LLC
(federally funded)
National Updated
semiannually
Notably, state laws regarding: access to naloxone,
Good Samaritan 911 immunity, PDMPs
administration, and regulation and reporting
Data download is a paid
feature.
See MonQcle
data maps
example here.
Merged at the state-level
with opioid-related
outcomes
Kaiser Family Foundation (KFF) data: Polling data on a variety of public health issues and opinions. Also compiles information from other secondary sources (e.g., CPS) to
provide state-level data on health indicators. http://www.kff.org/
KFF (private) National Varies Public opinion on opioid use; polling data from
public and medical officials; health insurance
coverage
No costs stated. Publicly
available
Access state
profiles here.
Merged with state-level
data on opioid-related
outcomes
Policy Surveillance Program (PSP): Program aiming to increase the use of policy surveillance and legal mapping as tools for improving the nation's health. Data from legal
mapping to understand the laws on a given topic and how those laws differ over time and across jurisdictions. http://lawatlas.org/
Temple National Updated Opioid policies and regulations across states No costs stated. Publicly Maps are Merged at the state level
University semiannually available. Historical data are available online with information on
LawAtlas Project not available or readily here. opioid-related outcomes
(private) downloadable for all policies
PDMP Training and Technical Assistance Center (TTAC) at Brandeis: The PDMP Training and Technical Assistance Center (PDMP TTAC) at Brandeis University provides a
wide range of services and resources to PDMP agencies, researchers, and other stakeholders in an effort to advance the effectiveness of PDMPs to combat misuse and abuse
of prescription drugs. http://www.pdmpassist.org/
TTAC @ Brandeis National Updated fairly Information on timing of state PDMP laws and No costs stated. Publicly See maps and Merged at the state level
(federally funded) regularly PDMP law components available. Historical data are
not available or readily
downloadable for all policies
tables of PDMPs
available here.
with information on
opioid-related outcomes
CDC Public Health Law Program (PHLP): Laws summarizing legal strategies used by states to address the misuse, abuse, and health impacts of prescription drugs.
https://www.cdc.gov/phlp/index.html
CDC (federal) National Not stated Time and dosage limit laws; physical exam
requirements; doctor shopping laws; patient
identification laws; pain management clinic
regulations; Good Samaritan laws
No costs stated. Publicly
available. Historical data are
not available or readily
downloadable for all policies
See state laws on
prescription drug
misuse and abuse
here.
Merged at the state level
with information on
opioid-related outcomes
90
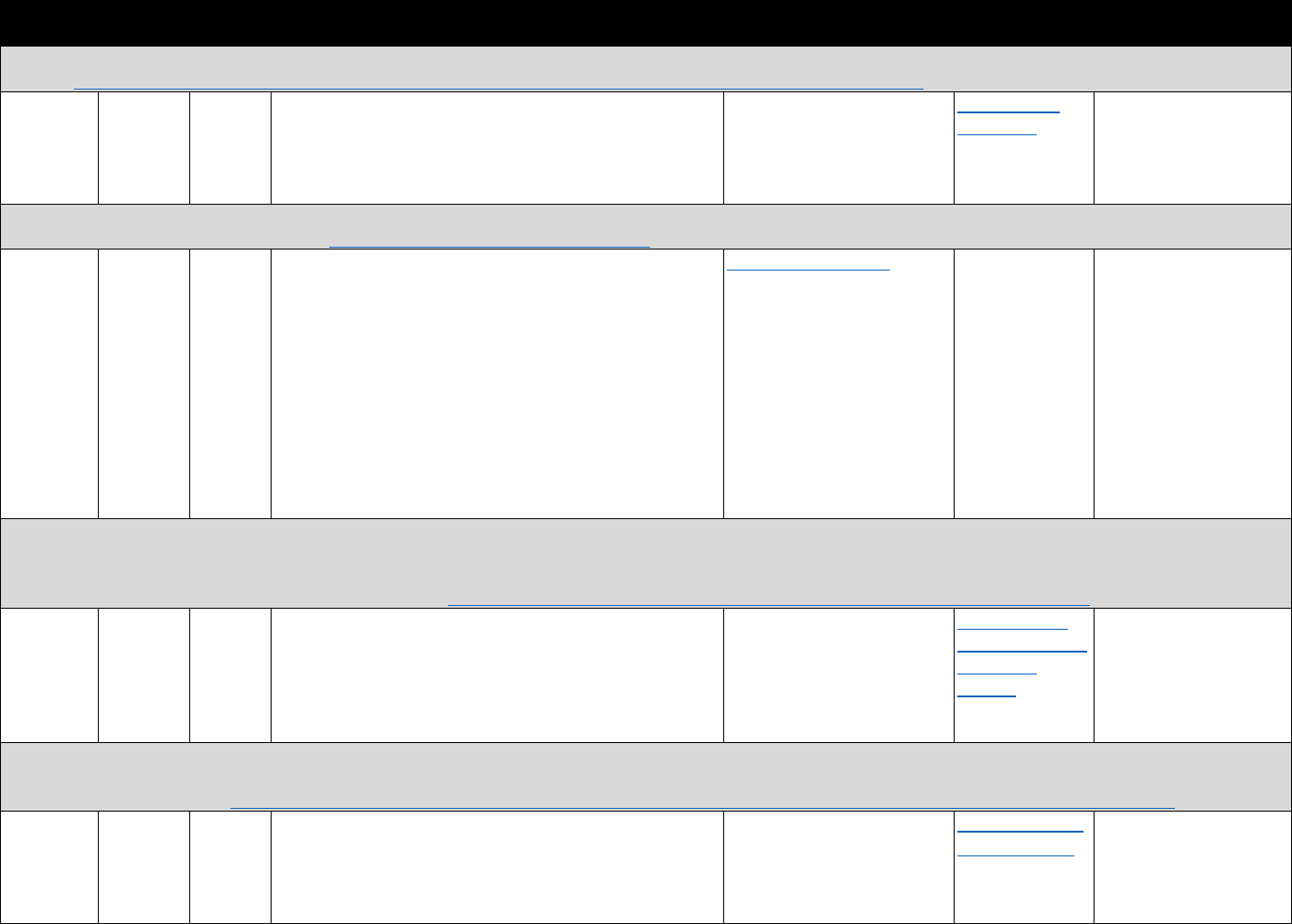
Agency Coverage Timing Measures Costs and Restrictions
Available
Analytics Linking Capability
SAMSHA buprenorphine physician treatment locator: SAMHSA tracks the number of DATA-Certified Physicians waivered to prescribe buprenorphine in each state and
territory. https://www.samhsa.gov/medication-assisted-treatment/physician-program-data/treatment-physician-locator
SAMHSA National Daily Number and location of DATA-Certified physicians; waiver No costs. Public use files are See counts by Merged with other zip
(federal) limits not a complete census of
providers, but a complete
census is available as
restricted-use files
state here. code–, county- or state-
level information
DEA Active Controlled Substances Act Registrants Database (ACSA): Contains a full list of addresses for physicians with DATA waivers, as well as a full list of practitioners
registered to handle controlled substances. https://classic.ntis.gov/products/dea-csa/
DEA;
distributed
by the
National
Technical
Information
Services of
the U.S.
Department
of
Commerce
(federal)
National Daily Number and location of DATA-Certified physicians See fee schedule here. Online access
available with fee
Merged with other
county- or state-level
information
Drug Abuse Warning Network (DAWN): DAWN is a public health surveillance system that monitors drug-related emergency department visits in the United States and for
select metropolitan areas. DAWN relies on a nationally representative sample of general, non-federal hospitals operating 24-hour emergency departments, with
oversampling of hospitals in selected metropolitan areas. In each participating hospital, emergency department medical records are reviewed retrospectively to find the
emergency department visits that involved recent drug use.
SAMHSA National Annual Opioid misuse and abuse–related emergency department No stated costs. DAWN was Online analysis Compared with other
(federal) visits; mortality data (only for subset of states). All types of
drugs are included. Alcohol is considered an illicit drug
when consumed by patients aged 20 or younger. For
patients over 21 years old, alcohol is reported only when it
is used in conjunction with other drugs.
discontinued in 2011, but
SAMHSA is developing other
sources of data on drug-
related emergency visits
provided through
ICPSR with
account
surveillance data sources
U.S. Food and Drug Administration (FDA) Adverse Event Reporting System: The FDA Adverse Event Reporting System (FAERS) is a database that contains information on
adverse event and medication error reports submitted to FDA. The database is designed to support the FDA's post-marketing safety surveillance program for drug and
therapeutic biologic products. https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070093.htm
FDA National Quarterly Reports of abuse-related adverse events. Contains detail on No stated costs. Public files See FAERS public None identified
(federal) product and substance with formulation- and composition-
specific differentiation
are available and individual
case safety reports can be
obtained by sending a FOIA
request to the FDA
dashboard here
.
91
Table A.6. Other National, State, and Local Secondary Data Sources
https://www.samhsa.gov/data/data-we-collect/dawn-drug-abuse-warning-network
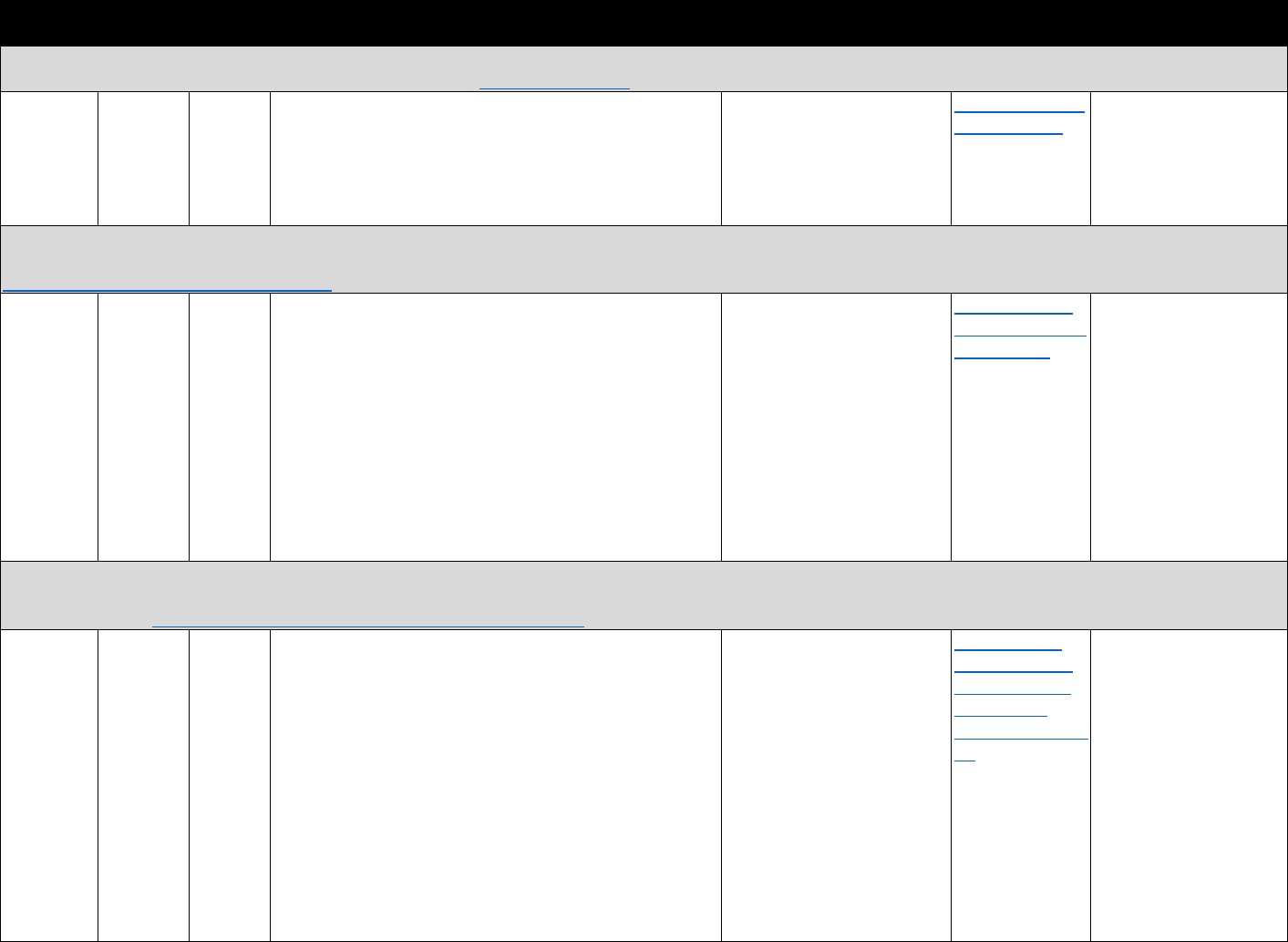
Agency Coverage Timing Measures Costs and Restrictions
Available
Analytics Linking Capability
National Emergency Medical Services Information System (NEMSIS): Provides data on EMS events for nearly all states. The consolidated data, while not a random sample
or census, is considered representative of national EMS activity. https://nemsis.org/
NHTSA
(Federal)
National
(49 states
as of 2016)
Annual Basic 911 call information about the scene of injury or
illness, medications administered (including naloxone), and
Emergency Medical Service (EMS) provider level, dispatch
call indicated overdose event, recorded overdose as injury
cause
No stated costs. Public-use
files must be requested;
certain variables are
restricted use and must go
through separate approval
process.
See data explorer
available here.
Has been merged with
mortality data based on
urbanicity
National Forensic Laboratory Information System (NFLIS): Drug cases investigated by the DEA. The data set provides information about chemistry of drugs seized by law
enforcement and analyzed by state, county, and volunteer forensic labs. Available for states, participating localities, and nationally.
https://www.deadiversion.usdoj.gov/nflis/
DEA
(federal)
National Monthly Drug identification results from drug cases submitted to
forensic laboratories
No stated costs. The private
site requires user accounts,
and security roles are
assigned to manage access to
its features, including the
Map Library, NFLIS Data Entry
Application, and Data Query
System. Only participating
laboratories and other DEA-
approved entities are granted
access to the Data Query
System
See information
about Data Query
System here.
None identified; linkages
at the state and
jurisdiction level likely
possible with access to
geocode identifiers
System to Retrieve Information from Drug Evidence (STRIDE): Data on drug exhibits remitted to DEA laboratories. The data set provides nationwide information on purity
and weight of each drug sample by month of seizure and total annual seizure weights by drug. Depending on the method of acquisition, information may be provided on
price of illicit drugs. https://www.dea.gov/resource-center/stride-data.shtml
DEA
(federal)
National Annual Street drug price by geographic area; street drug purity by
geographic area; volume of drug acquisitions (through
seizures, stings, purchases by undercover agents); product-
specific information
Some state-level annual
statistics available for
download online. More
detailed data can typically
only be obtained through a
FOIA request.
See state-level
annual statistics
here for heroin,
cocaine, and
methamphetamin
es.
Linkages at the state,
city, and metropolitan
statistical area level
possible with access to
geocode identifiers
92

Agency Coverage Timing Measures Costs and Restrictions
Available
Analytics Linking Capability
National Poison Data System (NPDS): Data reported by the American Association of Poison Control Centers members. Provides information on poison call conditions across
the United States, including number of exposure calls by drug/substance at state and national levels. http://www.aapcc.org/data-system/uses-npds-data/
American National Monthly Poison control calls related to opioids or other drugs by Fees vary depending on NPDS offers a Can be merged with
Association “intentional exposures” (includes abuse, misuse, and request and requesting variety of other state or county
of Poison suspected suicidal) or “intentional abuse exposures.” organization. AAPCC NPDS analytical data level information
Control Contains detail on product type/composition Data Request Policy requires products,
Centers certain levels of internal although costs
(federal) approval prior to agreement
execution
apply
Nationwide Inpatient Sample (NIS) and State Inpatient Databases (SID) from HCUP: The NIS is the largest publicly available all-payer inpatient health care database in the
US, providing national estimates of hospital inpatient stays. Weighted, it estimates more than 35 million hospitalizations nationally. The NIS is sampled from the SID, which
are the state inpatient databases that contribute to HCUP (currently 48 states participate in the SID). https://www.hcup-us.ahrq.gov/nisoverview.jsp and
https://www.hcup-us.ahrq.gov/sidoverview.jsp
HCUP, National or Annual Opioid-related inpatient stays for specific diagnosis; patient See database catalog for Online query Previously linked at the
AHRQ state- demographic characteristics; expected payment source; costs. All users, including system through metropolitan statistical
(federal) specific total charges purchasers and collaborators,
must complete the online
training and must read/sign
the DUA for state databases
HCUPnet
Opioid-specific
analytics
level to other data sets.
Hospital identifier
unavailable for all states
beginning with 2012 NIS
Nationwide Emergency Department Sample (NEDS) and State Emergency Department Databases (SEDD) through HCUP: NEDS is the largest all-payer emergency
department database in the United States, providing national estimates of hospital-based emergency department visits. Weighted, it estimates roughly 143 million
emergency department visits. NEDS is sampled from the SID and SEDD—the SEDD capture emergency visits at hospital-affiliated emergency departments not resulting in
hospitalization (currently 36 states participate in the SEDD). https://www.hcup-us.ahrq.gov/nedsoverview.jsp and https://www.hcup
-
us.ahrq.gov/db/state/sedddbdocumentation.jsp
HCUP, National or Annual Opioid-related emergency department stays for specific See database catalog for Online query Linked at the state-level
AHRQ state- diagnosis; patient demographic characteristics; expected costs. All users, including system through with other data. Hospital
(federal) specific payment source; total charges purchasers and collaborators,
must complete the online
training and must read/sign
the DUA for state databases
HCUPnet
Opioid-specific
analytics
identifiers permit linkage
to hospital inpatient
databases
National Addictions Vigilance Intervention and Prevention Program (NAVIPPRO): NAVIPPRO is a comprehensive risk-management system for prescription opioids and
other Schedule II or III therapeutic agents. Continuous and ‘‘real-time’’ data streams are subjected to temporal and spatiotemporal signal detection strategies, followed up
with signal verification. NAVIPPRO monitors two proprietary data sources (ASI-MV Connect and web-onformed services survey on prescription misuse) and several publicly
available data sources (FDA-AERS, DAWN Live!, AAPCC New Core System database). http://www.inflexxion.com/asi-mv
Inflexxion Most of Near-real Lifetime nonmedical opioid, heroin use; first-time Costs and access restrictions None identified Geographically detailed
(private) the United
States
time nonmedical opioid use, heroin initiates; past-year and -
month heroin use; nonmedical opioid use by product; route
of administration; lifetime and past-year nonfatal opioid
overdose; source of opioids
apply. Costs vary by request.
Propriety data set
information may support
linkages at aggregate
level
93
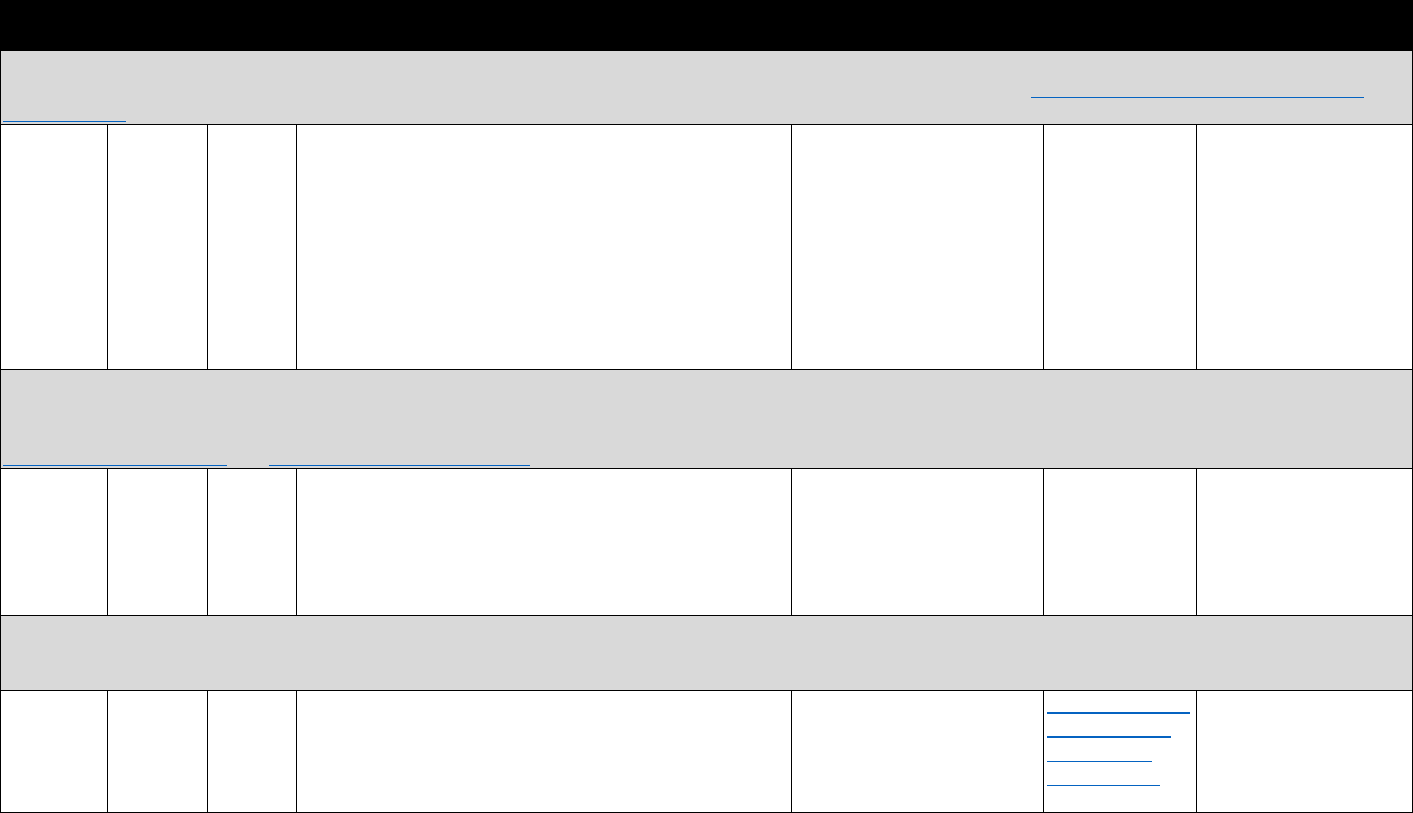
Agency Coverage Timing Measures Costs and Restrictions
Available
Analytics Linking Capability
Researched Abuse, Diversion and Addiction-Related Surveillance System (RADARS): RADARS consists of several programs: drug diversion, poison center, opioid treatment,
impaired health care worker, Survey of Key Informants, college survey, StreetRx (streetrx.com for street drug price) programs. https://www.radars.org/radars-system-
programs.html
Rocky
Mountain
Poison and
Drug
Center,
Denver
Health and
Hospital
Authority
(private)
Most of
the United
States
Near-real
time
Nonmedical opioid, heroin use; first-time nonmedical
opioid use, heroin initiates; past-year and -month heroin
use, nonmedical opioid use by product; measures of
diversion; street price of opioid products
Costs vary by request. Each
program in RADARS is
approved by the institutional
review board of the principal
investigator's institution
None identified—
will provide
customized
reports for a fee
Can be linked at the zip
code level to other
information
Harm Reduction Coalition (HRC) data on organizations providing naloxone to laypersons: In October 2010 and July 2014, the Harm Reduction Coalition emailed a survey to
staff in a sample of U.S. organizations known to distribute naloxone to laypersons. Surveys asked about year of program implementation and total amount of naloxone kits
distribution and number of individuals receiving training, as well as reported number of overdose reversals because of naloxone administration by program participants.
http://harmreduction.org/ also Link to recent report using data
Harm National Less than When the organization began operating; numbers of sites Costs not stated. Data not None identified Merged with state-level
Reduction annually or local programs providing naloxone kits; number of available publicly rates of overdose
Coalition (2010 and persons trained in overdose prevention and provided mortality
(private) 2014) naloxone kits; and number of reports of overdose reversals
(administration of naloxone by a trained layperson in the
event of an overdose)
Overdose education and naloxone distribution (OEND) program data: OEND programs serve as a source for naloxone distribution as well as training and education for
overdose response in communities throughout the United States. Several studies have used data from state- or site-specific programs to study research questions related to
overdose and overdose reversing drugs.
Varies, but State or Varies Number of trainings, overdose rescue behaviors, naloxone Data generally not available Example of OEND Linked with state- or
generally site- administrations, naloxone kits distributed publicly, although some state information for community-level
state specific agencies provide aggregate Rhode Island information on overdoses
agency statistics. available here and/or hospital
utilization rates
94
